In the pharmaceutical industry, water isn’t merely an ingredient to dissolve in, it’s a crucial one that directly affects drug safety efficiency, efficacy, and compliance. Two popular high-purity sources are reverse osmosis (RO) water as well as distilled water. But the question is: Is Reverse Osmosis Water The Same As Distilled Water? Let’s dive into the differences between them and their applications to ensure that your pharmaceutical processes are safe and efficient.
RO Water and Distilled Water Purification Methods
Reverse Osmosis (RO) Water–Membrane-Driven Purity
RO water is made by semipermeable membranes and high-pressure pumps, core components of RO Pure Water Systems. The process begins with raw water undergoing pretreatment-typically sand filtration to remove sediments and activated carbon filtration to eliminate chlorine and organic compounds. This shields RO membranes RO membrane from being damaged.
Then, high-pressure pumps push water into the membrane that acts as a molecular sieve. the membrane blocks between 97 and 99 percent of the dissolved heavy metals, salts bacteria, viruses and pyrogens. It also allows purified water molecules flow through. This is then stored inside a clean water tank, ready to use.
The most notable characteristics of RO water, as produced by RO Pure Water Systems, are the ability to produce continuously and high efficiency and scalability, making it a great choice for the needs of large-scale pharmaceuticals.

Distilled Water–Thermal Purification at Its Purest
Distilled water is based on heat to ensure the purity it requires. The water is boiled to produce steam that rises, and releases non-volatile contaminants such as bacteria, minerals, along with heavy metals. The steam is then condensed to liquid, creating water that is free of impurities. The process of batching is slower and energy intensive, yet it provides exceptional purity as the volatile organic compound (VOCs) are eliminated by the process of vaporization.
Distilled water’s main claim to fame is its complete absence of solids dissolved It is a preferred choice for those who require “zero impurity” standards.

Differences Between RO and Distilled Water for Pharm. Use
1. Purity and Contaminant Residues
Distilled water: Absolute purity has been its trademark. It has almost no minerals, ions, or VOCs, which is close to theoretical “pure water.” This makes it ideal for sterile formulations such as injectables in which even trace contaminants could threaten the safety of patients.
RO Water: Though it’s extremely pure, RO water can contain small quantities of mineral matter (depending on the membrane’s quality). In the case of most pharmaceutical use it is not a problem however it is a requirement for further treatment (e.g. electrodeionization also known as EDI) to be able to meet the highest standards in injectable drug use.
2. Conductivity
Conductivity-how well water conducts electricity-indicates ion levels, a critical factor in sensitive pharmaceutical processes:
Distilled water: It has virtually zero particles, its conductivity is around 0μs/cm. This is perfect for biopharmaceutical manufacturing (e.g. vaccines, vaccines monoclonal antibodies) in which ions can alter the active components.
RO Water:Typically it has conductivity less than 10μs/cm, which is acceptable to the standards of basic pharmaceutical water. Therefore, it’s not as suitable for ion sensitive applications.
3. Production Efficiency and Cost
Distilled Water: The speed of production in batches is limited and the energy needed to boil and condensate can increase costs. It is best suited for low-volume need, high-purity requirements (e.g. specialty injectables).
RO water: Automated continuous production via RO Pure Water Systems decreases the energy and labor costs. Its capacity makes it affordable for large-scale applications such as cleaning of equipment as well as diluting the oral formulas.
4. Regulatory Compliance
Pharmaceutical water should be aligned with the global pharmacopeias.
Distilled water: Conforms to USP Standards and European standards of “water for injection” (WFI) which is the gold standard for sterile medications. It is a direct use in injections and infusions.
RO water: by itself, RO water isn’t always in line with WFI standards. In reality, it’s used as a pretreatment method, reducing the strain on subsequent process of distillation and EDI systems in order to achieve WFI-quality.
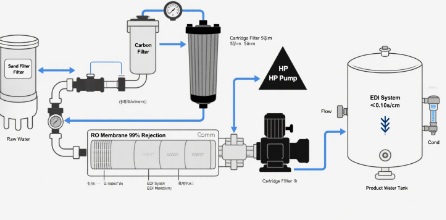
Choosing Between RO and Distilled Water in Pharmaceuticals
When to Use Distilled Water
Sterile formulations like injections, infusions, as well as ophthalmic solutions require no pyrogens or contaminants which is why distilled water offers this. Biopharmaceuticals such as the use of vaccines as well as gene therapies, are tolerant to ions. Therefore, the ultra-low conductivity of distilled water stops destabilization. Certain regions’ pharmacopeial regulations expressly require distilled water to be used to provide WFI as stated in local Pharmacopeias.
When RO Water Shines
Non-sterile medications such as chewable tablets and topical creams syrups, and topical creams have lower purity requirements and RO water, perhaps with EDI can be used to meet the requirements. Cleaning equipment requires large amounts of water to clean tanks pipes, pipes and filling lines and RO Pure Water Systems’ constant supply guarantees effectiveness. Utilizing RO water as a prelude to distillation or EDI as a pretreatment for water that is high-purity helps reduce energy consumption and extends the life of equipment.
| Factor | Distilled Water | RO Water |
| Purity | Extremely high (no residues) | High (97-99% desalination) |
| Conductivity | ~0μs/cm | ≤10μs/cm |
| Production | Batch, slow, energy-heavy | Continuous, efficient, scalable |
| Cost | Higher | Lower |
| Ideal Uses | Injectables, biopharmaceuticals | Oral drugs, cleaning, pretreatment |

Final Thoughts
RO water as well as distilled water aren’t interchangeable in the field of pharmaceuticals. Distilled water excels in high-risk small-scale applications that require absolute purity. On the other hand, RO water derived from RO Pure Water Systems is an excellent choice for large-scale, cost-effective requirements. For a lot of operations using a hybrid approach-using RO water from the RO pure water systems for pretreatment as well as distillation to purify the final product- strikes the ideal balance between efficiency, purity and conformity.
By ensuring that your water choices are aligned with the pharmacopeial standard and requirements for process, as well as production scale, you can ensure safety of your drugs, compliance with regulatory requirements, along with operational efficiency. Choose wisely, with tools like Molewater’s system supporting your efforts—your products, and patients, depend on it.








