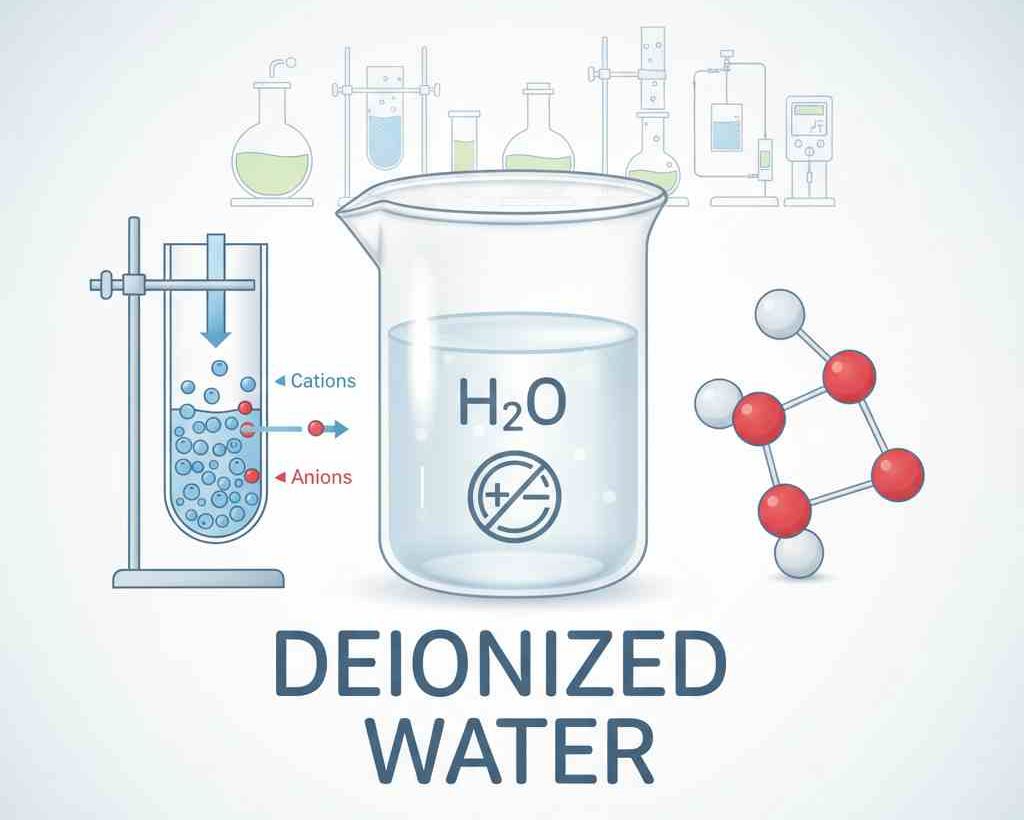
Deionized water is a kind of high-purity water obtained by removing dissolved ionic impurities from water. These dissolved ions include minerals and salts such as calcium, magnesium, sodium, chlorine and sulfate. Deionized water is different from ordinary tap water or distilled water and contains almost no impurities. Therefore, it has extremely high application value in both laboratory and industrial scenarios.
In the laboratory, it is often used for chemical experiments, preparing buffers, dissolving chemical reagents and cleaning experimental equipment. In the industrial field, high-purity water can prevent precision equipment from scaling or corroding, thereby extending the service life of the equipment and improving product quality.
By understanding the characteristics of deionized water, we can choose the usage method more scientifically, avoid experimental errors, and at the same time reduce equipment maintenance costs in industry.
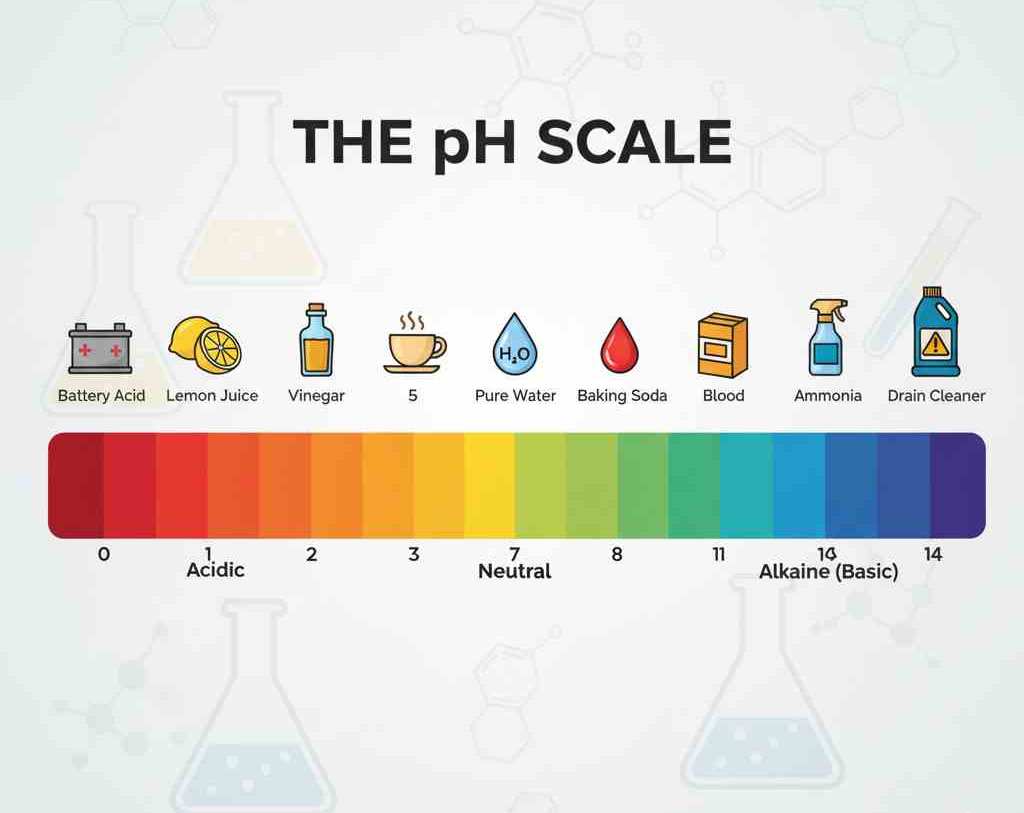
What Is The pH Of Deionized Water?
When deionized water is freshly prepared, its pH is close to neutral, approximately 7. This means that the concentrations of hydrogen ions and hydroxide ions in water are basically equal. However, due to the high purity of deionized water, it readily absorbs carbon dioxide from the air. When carbon dioxide in the air dissolves into water, it forms trace amounts of carbonic acid, slightly lowering the water’s pH level. This usually does not exceed around 6.5, but it may have an impact in some experiments that are sensitive to acids and bases.
Therefore, the usage environment and storage method of deionized water are extremely crucial. In operations that require strict control of pH levels, it is best to confirm the water quality before use to avoid experimental errors caused by improper storage.
The Method Of Making Deionized Water
The preparation of deionized water relies on ion exchange technology. During this process, water flows through cation exchange resin and anion exchange resin. The cation resin adsorbs positive ions in the water, while the anion resin adsorbs negative ions, thereby removing impurities from the water.
Modern preparation methods usually combine reverse osmosis technology. Reverse osmosis can first remove most of the dissolved salts, suspended particles and microorganisms in water, and then further enhance the purity of water through ion exchange. This can ensure that the final water obtained contains almost no impurities, making it suitable for high-demand experimental and industrial applications.
It is worth noting that deionized water of different grades varies in purity. The water commonly used in laboratories usually reaches a level where the vast majority of ions have been removed, while the water used in semiconductor, pharmaceutical or high-precision industrial production requires a higher purity standard.
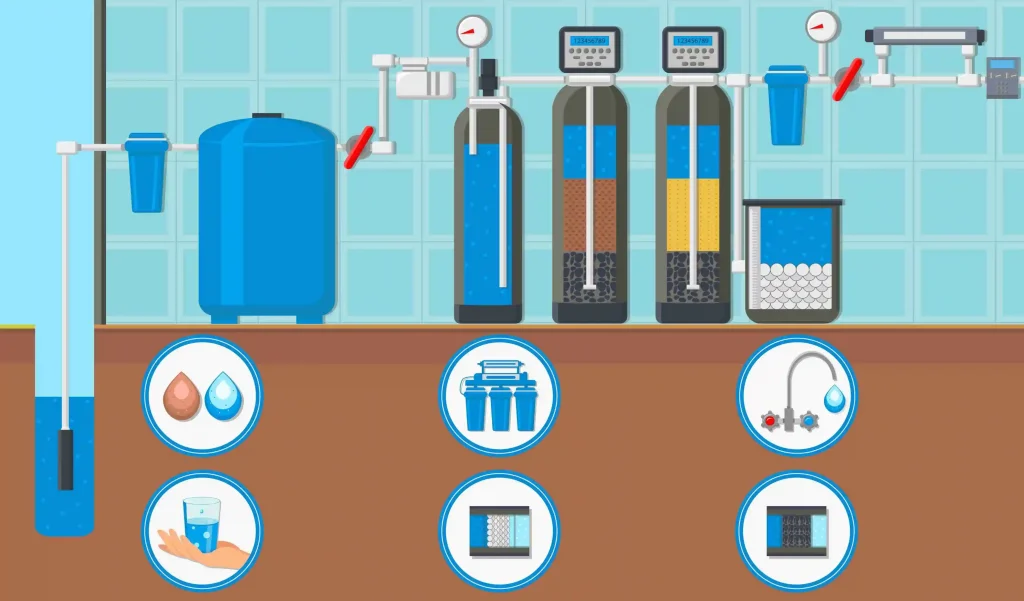
How Does a Deionized Water System Work?
A complete deionized water system is usually composed of pretreatment, ion exchange units, post-treatment and circulation systems.
- Pretreatment: Its main function is to remove suspended particles, residual chlorine and some organic matter from water, reducing the burden on the ion exchange unit.
- Ion exchange unit: Cation resin adsorbs positive ions in water, such as calcium, magnesium and sodium, while anion resin adsorbs negative ions, such as chloride ions and sulfate ions. Through this process, most of the dissolved ions in the water are removed.
- Post-treatment: Mixed bed resin or activated carbon is usually used to further improve water quality and ensure that the water reaches a high-purity level.
- Circulation system: Ensures the continuous circulation of water flow, enabling the system to provide stable high-purity water for a long time.
This system is widely used in laboratories and industries, ensuring the stability of water quality each time it is taken, thereby guaranteeing the reliability of experiments or production processes. For laboratories that require a stable supply of high-purity water, choosing a reliable deionized water system is of vital importance.
Molewater Lab Deionized Water System offers a fully automated design, integrating pretreatment, ion exchange and post-treatment. It can continuously supply high-purity water to meet various experimental requirements. Meanwhile, this system is easy to operate and has a long maintenance cycle, making it highly suitable for small and medium-sized laboratories and research and development units.
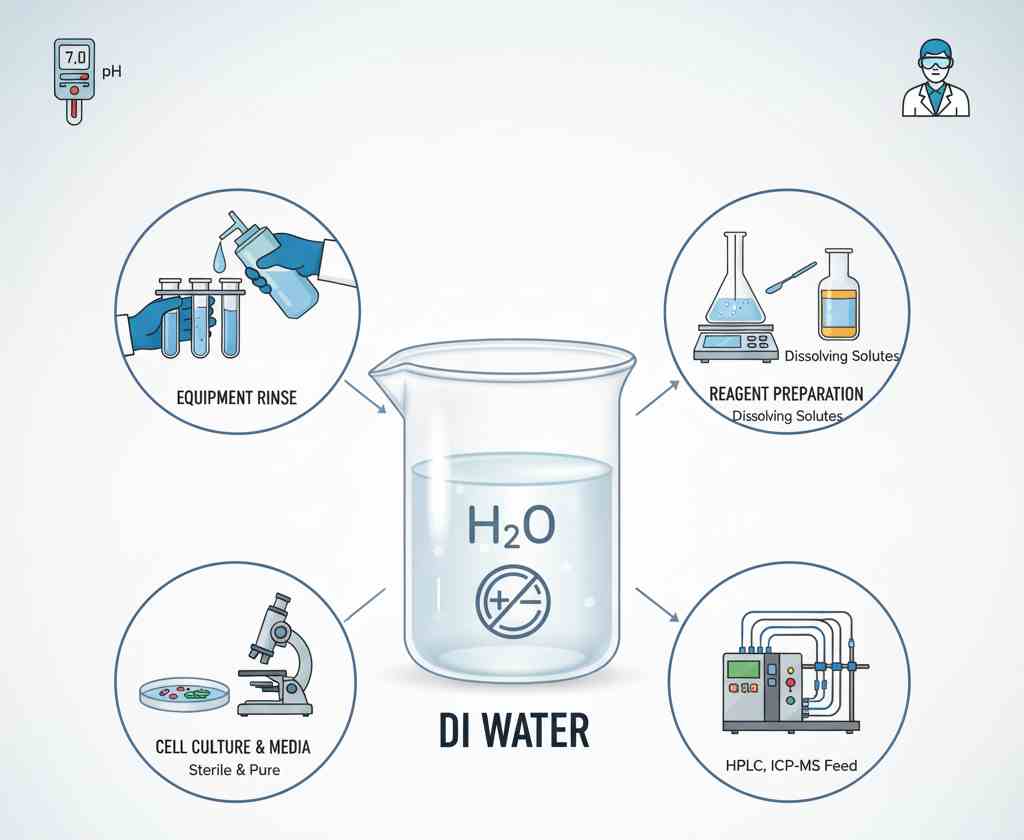
What Is Deionized Water Used For?
Deionized water, due to its high purity and low conductivity, has a wide range of uses in laboratories and industries.
Laboratory
- Chemical analysis: High-purity water can prevent impurities from interfering with the analysis results.
- Solution preparation: When preparing buffer solutions and reagent solutions, deionized water can ensure the accuracy of the experiment.
- Instrument cleaning: It is used in the cleaning of samples from spectrometers, chromatographs or microscopes to prevent residues from affecting the performance of the instruments.
- Medium preparation: Pure water is used in microbiological experiments or cell culture to ensure the purity of the experimental environment.
Industrial
- Electronic manufacturing: High-purity water can be used for chip cleaning and printed circuit board rinsing to prevent conductive impurities from affecting electronic performance.
- Pharmaceutical production: Deionized water is used for solution preparation and equipment cleaning to ensure the quality of drugs.
- Boiler make-up water: Reduces scale formation and corrosion, and extends equipment service life.
- Food and beverage processing: Ensure product safety and avoid the impact of mineral and ion residues on taste and stability.
Through these applications, the significance and wide applicability of deionized water can be seen. It not only ensures the accuracy of experiments but also provides a stable water source for industrial production.
Precautions for Use
Although deionized water is pure, the following points still need to be noted when using and storing it:
- Avoid prolonged exposure to air: Carbon dioxide in the air can alter the pH level of water, thereby affecting experiments or industrial processes.
- Use clean containers: Impurities or microorganisms remaining in the containers may contaminate the water quality and affect the usage effect.
- Regular maintenance of equipment: Ion exchange resins will gradually become saturated with use and need to be replaced in a timely manner to ensure the long-term stability of water quality.
- Avoid high temperatures and sunlight: High temperatures or sunlight may promote the growth of microorganisms or alter water quality, affecting the performance of pure water.
By following these methods, it can be ensured that deionized water performs at its best in laboratory and industrial applications, reducing equipment failures or experimental deviations.
Final Thoughts
Deionized water, with its high purity and low impurity characteristics, has become an indispensable water source in laboratories and industries. From basic concepts, acid-base properties, to preparation principles, system operation modes, and then to application and maintenance methods, laboratory and industrial users can use high-purity water more scientifically.
Whether it is precision experiments, electronic manufacturing, pharmaceutical production or boiler make-up water, high-purity water provides reliable guarantees for operations. In experiments and production, the rational use and maintenance of deionized water systems are the keys to ensuring stability and high efficiency.






