If you’ve ever wondered why reverse osmosis (RO) systems are used for drinking water or why a wilted lettuce leaf perks up in a bowl of water you’re not alone. These everyday moments tie back to osmosis and reverse osmosis two processes that shape how water moves and cleans. This article breaks down their key differences so you understand what they do and when they matter for your daily life.
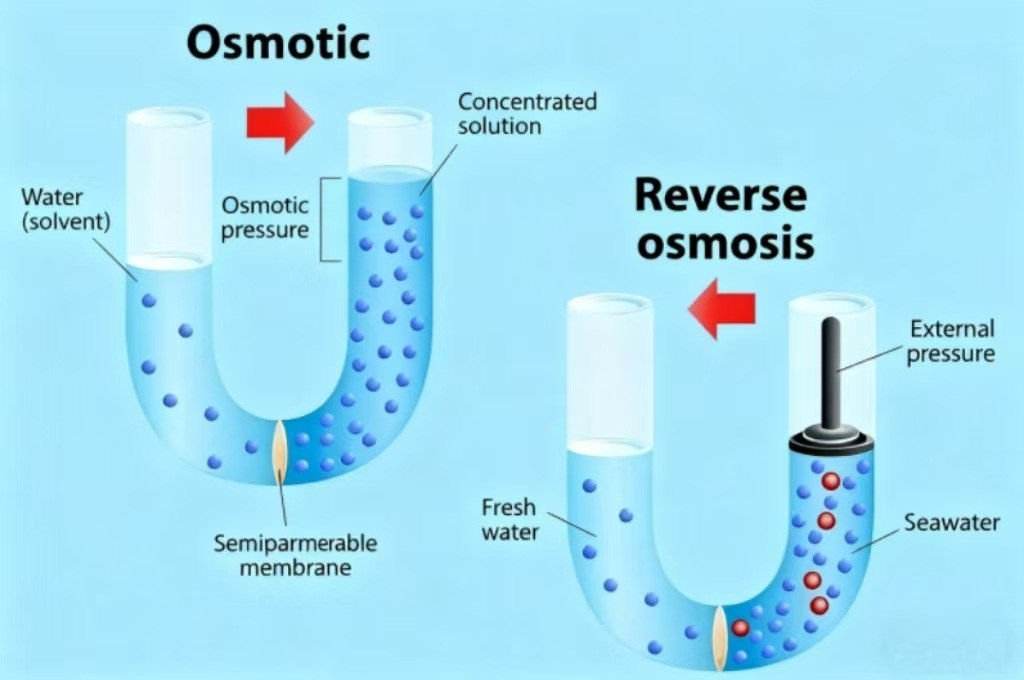
Understanding Osmosis and Reverse Osmosis
You don’t have to be a science expert to understand these ideas. Let’s look at examples that you’ll be able to recognize.
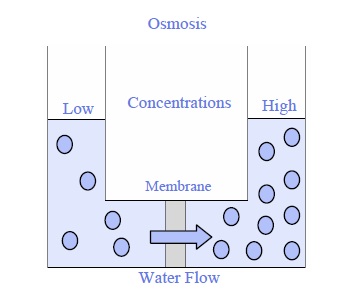
Osmosis is a nature method to balance water. It occurs when water moves from a place with less chemicals dissolved, like pure water, to an area with higher concentrations of dissolve substances like saltwater, or inside the cell of a plant. This movement continues until both areas have a similar concentration of dissolved stuff. Think about that wilted lettuce leaf: when you submerge it in water osmosis pulls water into the leaf’s cells making it crisp again. It’s a natural passive process that needs no extra help.
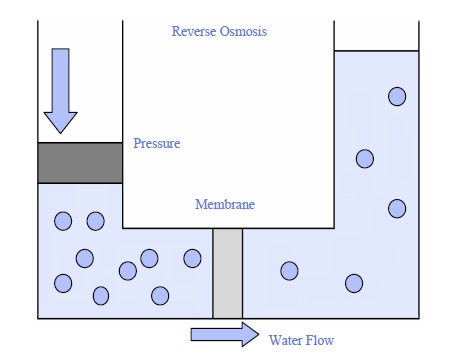
Reverse osmosis is the opposite. It’s a human-made process that pushes water against its natural osmosis direction—from an area with more dissolved substances to one with fewer. To make this happen we use pressure (often from a pump). This force traps impurities like salt chemicals or bacteria on one side while allowing clean water to pass through. You’ll find this technology in home water filters where it turns tap water into safe drinking water by removing contaminants.
The key takeaway? Osmosis is nature’s balance act, while reverse Osmosis is the method we employ to use the technology of today to “trick” that process for practical purposes like water purification.
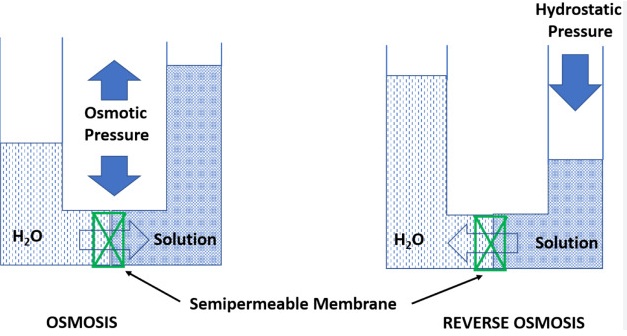
Key Differences Between Osmosis and Reverse Osmosis
Understanding the difference can help to understand why each process is important. Let’s look at the key elements.
Direction of Water Movement
Many people get confused over which method of water flow. Here’s a quick method to remember: Osmosis is a natural process that follows the lead of nature. Water flows naturally from low-solute zones (less chemicals or salt) to areas with high sulfate (more salt or chemical) to achieve equilibrium. There is no pressure or energy required, it happens naturally.
Reverse osmosis impedes that natural flow. Water flows from high-solute regions to areas with lower sulfate levels, however only when we exert pressure. Without the additional force, the water will not be able to move this way by itself. An effective way to remember? Osmosis refers to “with nature” and reverse Osmosis can be described as “against nature.”
Purpose
Osmosis is the natural maintenance tool. It helps keep the roots of plants well-hydrated, which means your garden will grow and assists your body in absorbing water from your food. It is also involved in the preservation of food. When you create pickles or salted meat, the process of osmosis removes water from bacteria to stop their growth and stopping food from rotting.
Reverse osmosis is a water-cleaning trick. It shoves tap water through a super-tight filter so lead, chlorine and other junk stay behind and only clean water comes out. People mount little RO units under the kitchen sink for drinking water, stick them on fish tanks to keep fish safe from chemicals, and build giant versions on the coast that turn ocean saltwater into fresh drinking water for towns that don’t have enough rivers or wells.
Use of Energy
One of the most common concerns is whether these processes will cost an amount of money to run. Osmosis does not require any energy whatsoever. It’s a process that is based on nature’s balance. You don’t have to plug into a plant or bowl of water in order to make osmosis occur. It happens on its own.
Reverse osmosis is a process that requires energy, mostly in the form electricity. The pressure needed to force fluid against the natural stream is from a small pump that is found in the majority of systems. RO systems at home don’t consume any energy, typically less than the typical light bulb, but there is a major difference from the osmosis method.
Applications of Osmosis vs. Reverse Osmosis
Understanding how these processes function every day helps to make them more understandable.
Applications of Osmosis
Osmosis has a greater impact on your life than you believe. When you make espresso in the early morning,, osmosis aids in the absorption of flavor by water from the coffee beans that are ground. If you do not water your plants in the house, osmosis slows down and leaves the plant wilting. Watering it will restart osmosis and can revive the plant. Your body too uses osmosis as it assists in moving water out of your digestive tract into your cells to keep your body fully hydrated.
Applications of Reverse Osmosis
Reverse Osmosis is a great option to purify water. RO systems at home are a popular choice for families who wish to stay away from bottled water or aren’t comfortable with the water from their tap. These systems remove harmful substances such as lead arsenic, chlorine and other making drinking water more safe. In the hobby of aquariums, reverse osmosis water is an absolute necessity to remove the minerals and other chemicals in tap water to prevent algae growing and keeping fish healthy. On a larger scale, desalination plants in areas such as Dubai and California make use of reverse osmosis to supply freshwater for millions. It’s also widely used in industries that demand high-purity water, such as pharmaceuticals and electronics–pharmaceutical facilities use it to purify water for formulating medications (ensuring compliance with strict health standards).

Role of Ion Exchange in Reverse Osmosis Technology
You may have seen “RO + ion exchange” systems and wondered what an ion exchange is. It’s a step in the second stage of cleaning which works in conjunction with reverse osmosis to enhance the quality of water.
Reverse osmosis is a method of trapping massive impurities such as salt bacteria and chemical compounds, but it could also leave out tiny minerals dissolved like magnesium and calcium. These minerals can cause “hard water” which leaves spots on dishes, clogs pipes and causes soap to foam poor. Ion exchange helps remove these minerals by combining these ions for harmless ones such as sodium. It makes water “soft” and more pleasant to drink.
Ion exchange isn’t a requirement to reverse osmosis, but it can be beneficial if you have trouble in the face of hard water. It’s a tiny extra step, but the end outcome is softer and cleaner water that’s ideal for drinking cooking as well as household chores.
Do You Need to “Choose” Between Them?
A lot of people wonder whether one can be considered to be “better” than the other however the reality is that you don’t need to decide. They have completely different functions.
Osmosis is an organic process you can’t renounce. It’s vital for the growth of plants, as well as food preservation. It’s not necessary to “use” osmosis–it works around you all the time.
Reverse osmosis is one of the tools that you can use in the event that you require it. Think about using an RO device if need safe drinking water, especially in the event that your tap water contains known contaminants. It’s also a good option for those who have hard water and wish to lessen the buildup of minerals or if you have saltwater fish that require clean water.
If you don’t meet these requirements, you may not require reverse osmosis all. Osmosis will continue to function in a natural way, keeping your plants active and your body hydrated, without any effort on your part.
Final Words
Osmosis and reverse-osmosis are two different sides of one coin: Osmosis is nature’s natural passive water balancer which sustains both health and plants as well as reverse osmosis, which is a technology that uses energy to purify secure water. If you are looking for secure RO solutions, look into Molewater’s Reverse Osmosis Direct Drinking Water Treatment Plant that is built using the most reliable high-purity water technology. Both systems keep things running smoothly – one natural and one that is technological.





