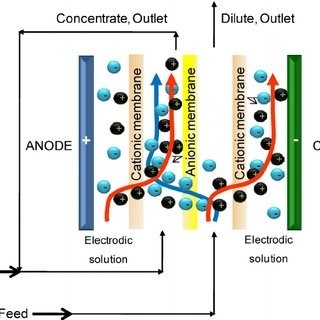
Electrodeionization (EDI), as the foundation of pharmaceutical pure water production, facilitates deep desalination via electric field without chemical regenerants and thus ensures a continuous water supply. But downtime for an EDI unit is fatal to pharmaceutical firms: production stops (causing heavy economic losses), product quality issues arise due to fluctuations in water flow rates, GMP compliance is violated and regulatory penalties or trust crises could arise, so developing an efficient maintenance system for regulatory compliance and risk management must be established as soon as possible for continuity and risk control purposes.

Pretreatment System Maintenance First Line of Defense for EDI
The pretreatment system is the foundation for EDI equipment’s stable operation. Its core function is to remove impurities like suspended solids, colloids, organics, and residual chlorine from raw water, preventing contaminants from entering subsequent units and causing blockages or damage—truly the “first line of defense” for EDI systems.
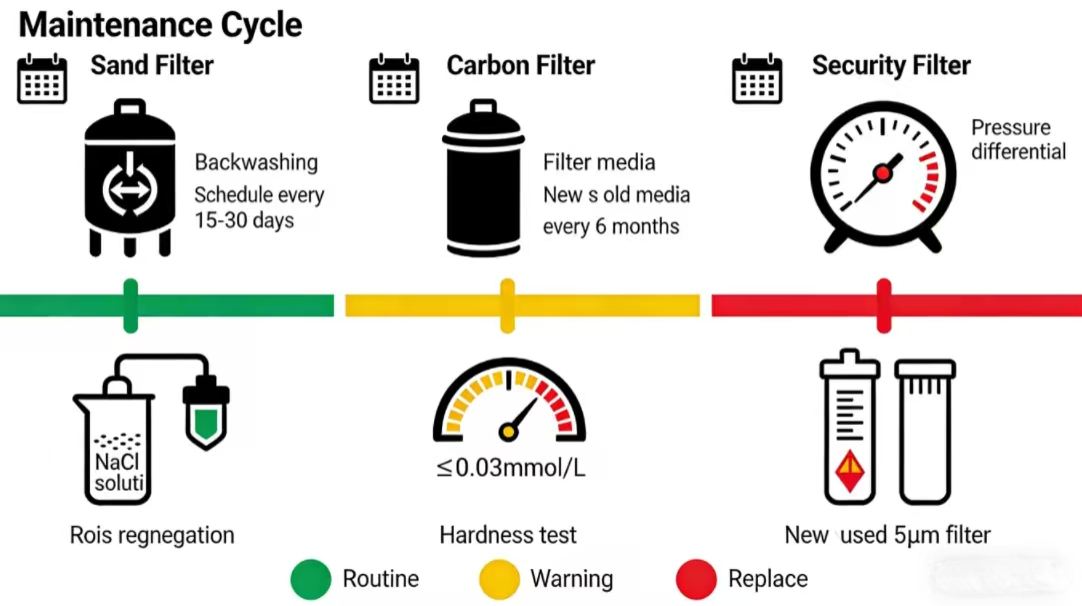
For sand and carbon filter maintenance, adhere to strict cycles. Sand filters are designed to remove large suspended solids while carbon filters absorb residual chlorine and partial organics from the environment. Both require backwashing every 15-30 days in order to flush filter media contaminants through reverse flow; every 6 months or so replace filter media in order to maintain efficiency. Shorten backwashing cycles if raw water turbidity is high to avoid caking.
Softener maintenance focuses on regeneration and hardness control. Pharmaceutical EDI demands feed water hardness ≤0.03mmol/L to prevent calcium-magnesium scaling that clogs fresh water channels. Regenerate resin with sodium chloride solution based on inlet hardness and output, testing effluent hardness before operation.
As the final pretreatment barrier, the security filter uses 5μm precision elements to trap fine impurities. Replace elements immediately when pressure difference exceeds 0.5bar to avoid rupture and contamination. Always check the seal’s integrity regularly to ensure that there is no any bypass.
Monitoring of water quality in real-time is crucial, and includes key indicators being the turbidity ≤0.1NTU and residual chlorine ≤0.05mg/L and suspended solids of less than 1ppm. Examine carbon filters and replace activated carbon in the event that residual chlorine is too high; increase backwashing of sand filters for high quality suspended solids or high turbidity. make sure you have qualified EDI feeding water.
RO System Co-Maintenance Qualified Feed Water for EDI
As a critical pre-unit for EDI equipment, the RO (Reverse Osmosis) system’s permeate serves as EDI’s feed water, with its quality directly impacting EDI membrane efficiency and lifespan. Thus, coordinated RO maintenance is vital to ensure stable, qualified water supply for EDI.
Pollution warning and chemical cleaning are core to RO membrane maintenance.
Clean when permeate output drops by 10% or pressure difference increases by 15%. Employ citric acid for inorganic scaling such as calcium carbonate build-up and sodium hydroxide for organic/microbial contamination; use proper concentration, temperature, rinsing time and chemical temperature controls to avoid corrosion – then thoroughly rinse using pure water afterward.
Comply with RO membrane replacement and storage standards. Under normal circumstances, membranes should last 1-3 years (shorter with inadequate feed water or insufficient maintenance).

Replace when permeate salinity spikes persist post-cleaning. Store idle membranes sealed in sodium bisulfite solution to prevent drying and oxidation.Parameter matching between RO and EDI is also crucial. Control RO permeate salinity ≤50μS/cm to avoid overloading EDI and exacerbating membrane polarization. Coordinate RO and EDI pressures to prevent shock damage from fluctuations. Synchronously monitor RO water quality and EDI operating parameters for optimal compatibility.
Practical Maintenance for EDI Core Modules
The EDI core module is the equipment’s heart, with its maintenance quality directly determining water purity and operational stability. Maintenance focuses on four key aspects: chemical cleaning, parameter monitoring, pollution treatment, and replacement standards, ensuring scientific and compliant operations.
Regular chemical cleaning of an EDI membrane is critical for its longevity. Over time, trace impurities from pretreatment, scaling and organic adsorption cause gradual contamination that compromises water purity while increasing energy consumption. Switch between citric acid for inorganic scaling and sodium hydroxide with surfactant for organic/microbial pollution every three months for cleaning purposes – maintaining temperatures at 25-30 ℃with adjustment depending upon contamination level (usually 30-60 minutes of cleaning time per session), stirring continuously throughout for full contact between each step in cleaning cycle.
Strictly monitor operating parameters to avoid membrane deformation and temperature exceed 45 ℃ (to prevent resin aging) as well as current of between 0.5-1.5 A (for efficient desalination and no risk of polarization/hydrogen formation), using real-time online meters to track changes and make necessary corrections if any anomalies arise.
Accurately identify pollution types for targeted treatment:
- scaling (decreased resistance, increased pressure) addressed by acid cleaning;
- organic contamination (viscous deposits) by alkali cleaning;
- microbial pollution (bacteria exceeding limits) by disinfection + alkali cleaning.
Judge via inlet/outlet water quality, parameter changes, or membrane disassembly if needed.
Control module replacement and installation strictly. Replace membranes when conductivity persists ≥0.5μS/cm post-cleaning (indicating severe resin aging). Ensure tight pipe connections during installation, clean electrode plates to remove oxide layers, and conduct trial operation to verify water quality and pressure meet pharmaceutical standards.
Pharmaceutical-Specific Maintenance Compliance & Safety
The pharmaceutical industry’s uniqueness requires EDI equipment maintenance to balance operational stability and compliance, while strengthening safety controls to avoid quality risks and accidents from improper operations.
GMP-compliant maintenance record management is the core of compliance. Per GMP standards, EDI operational data, maintenance activities, and consumable replacements must be fully traceable and archived—including maintenance dates, operators, tasks, and test results. Create dual electronic and paper filing systems to maintain full traceability for regulatory inspections. Include equipment IDs, maintenance cycles and compliance criteria as part of this recordkeeping.
Microbial contamination control is of great importance when producing pharmaceutical grade water for consumption, with pharmaceutical standards mandating sterility being met through EDI treatment of its effluents. UV disinfection should be utilized during pretreatment to kill raw water microbes; biocidal cleaners should then be added during membrane cleaning to further inhibit growth and ensure bacteria counts do not exceed 10CFU/mL as required by pharmaceutical standards.
Routine corrosion prevention of electrodes (often precious metals) is essential. Water quality fluctuations and abnormal currents are known to lead to corrosion of EDI electrodes, so inspect them regularly for signs of oxidation or peeling and maintain an inlet water pH between 7-8 for best results. Keep connections dry and clean to avoid leakage or poor contact between electrodes.
System shutdown protection ensures long-term stability. For periods of downtime lasting 30 or more days: wet preservation (submerge membranes in pure water and replace regularly) can be used for short term applications; when used for extended downtimes use dry preservation instead. When dealing with long periods, use wet preservation for short duration periods followed by dry preservation for longer term needs (rinse them with pure water then dry with nitrogen before sealing in dry environment), periodically inspect equipment during shutdown to check for moisture, contamination or resin aging issues before sealing or sealing dry storage environments
Conclusion
The stable operation of EDI equipment in the pharmaceutical industry relies on a scientific and standardized maintenance system—from the basic protection of pretreatment systems to the coordinated cooperation of RO systems and the precise maintenance of core modules. Each link directly impacts operational efficiency, equipment lifespan, and water quality. Practice proves that standardized maintenance can extend EDI service life by over 30% and reduce troubleshooting costs by 50%, significantly enhancing operational economy.
Pharmaceutical enterprises know that EDI maintenance is both an administrative and compliance responsibility. If you require high-quality EDI equipment that supports both production and compliance goals, Molewater provides excellent performance, durability, and industry expertise to make an excellent choice.