Guatemala 750 LPH Two-Stage RO Pharmaceutical Water System with Full SS316L
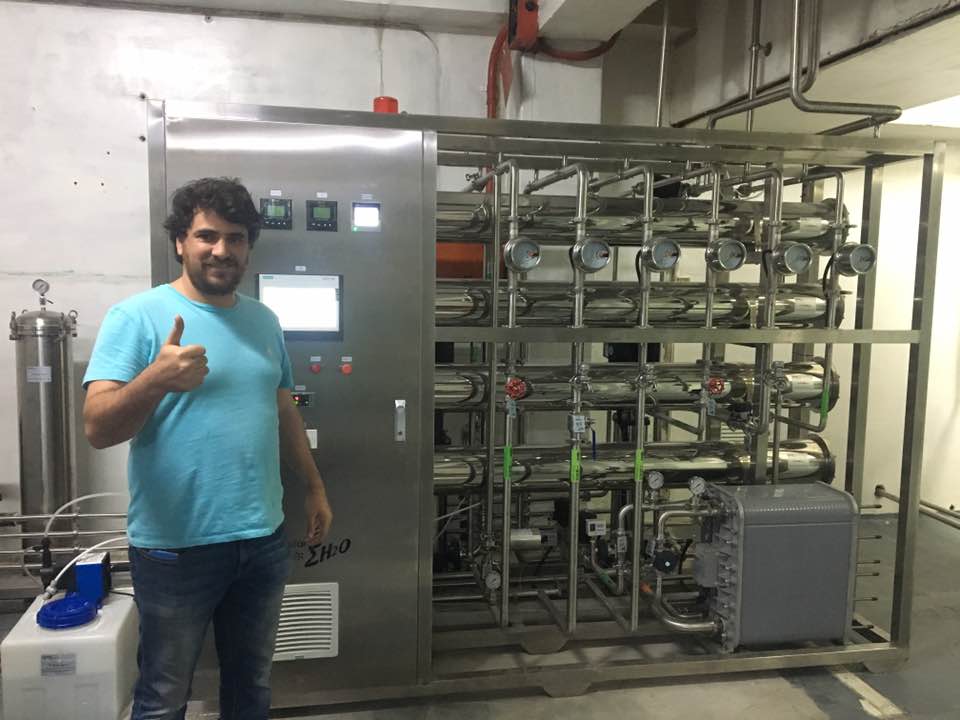
This 750Lph two-stage reverse osmosis pharmaceutical pure water equipment is specially designed for the pharmaceutical, biological products and high-end medical device industries in Guatemala and Central America, dedicated to providing water for injection (WFI) or high-purity purified water that meets the strict requirements of international pharmacopoeias. The system adopts a core process combination of “full-process SS316L stainless steel material” and “two-stage reverse osmosis + electro-deionization (EDI)/fine treatment”, ensuring the stable production of high-quality process water with low endotoxins and low microorganisms even in tropical climate conditions. It is suitable for high-demand links such as sterile preparation production, biological fermentation, cleaning and validation, and laboratories.
System process Introduction: This system is designed to balance high water production capacity and reliability in extreme water quality, with a precise and complete process flow

Enhanced pretreatment unit In response to the complex raw water conditions that Guatemala may encounter (such as high hardness, high organic matter or seasonal turbidity variations), the system is equipped with fully automatic multi-media filters, activated carbon filters and a dual-tank softening system (one in use and one as backup or alternately regenerated), and all use SS316L stainless steel tanks to effectively remove suspended solids, colloids, residual chlorine, organic matter and calcium and magnesium ions Provide the best and most stable feed water guarantee for reverse osmosis membranes and extend the service life of core components.

Core dual-stage reverse osmosis purification module: It adopts a highly efficient dual-stage RO design. The first stage RO deeply desalinates, and the second stage RO refines and polishes the water produced by the first stage. The overall desalination rate of the system is stable at ≥99.5%, and it can efficiently remove ions, organic matter, colloids and microorganisms. The complete set of RO pressure pipelines, membrane housings and frames are all made of SS316L stainless steel to ensure long-term structural integrity under the potential corrosion risk of high salt content or chloride ions.

Deep deionization and fine treatment unit: The secondary RO product water enters the continuous electro-deionization (EDI) module, where the resistivity of the product water is continuously increased to 10-18 MΩ·cm without the need for chemical regeneration. Subsequently, it undergoes ultimate microbial and particulate matter control through a 254nm ultraviolet sterilizer and an optional 0.22μm precision filter. The final produced water enters the SS316L stainless steel sanitary storage tank and the circulation distribution system. The entire pipeline process is treated with automatic welding and electrolytic polishing (EP), and integrates a superheated water or pure steam disinfection function to prevent secondary pollution during the distribution process.
Core processing system
The complete SS316L stainless steel structure: from the pretreatment tank body, core module frame, all process pipelines, valves, pump body contact parts to the final storage tank and distribution system, all are made of SS316L ultra-low carbon austenitic stainless steel. This material features excellent corrosion resistance (especially against chloride stress corrosion), high surface finish and outstanding mechanical properties. It fully complies with international hygiene equipment standards such as cGMP, FDA and ASME BPE, and is suitable for the environmental challenges of high temperature and high humidity in Guatemala.
High-capacity and stable operation design: In response to the continuous water production demand of 750Lph, the system adopts industrial-grade high-pressure pumps, high-flow membrane elements and an optimized recovery rate design. The control logic supports smooth start and stop, low-pressure flushing and automatic chemical cleaning (CIP), ensuring the high efficiency and stability of the system during long-term continuous operation.
Fully automatic intelligent control and monitoring system: Integrating a control center centered on PLC and high-resolution touch screen (HMI), it comprehensively monitors and records key parameters such as raw water quality, conductivity/resistivity of produced water at each stage, working pressure, temperature, flow rate, UV intensity, and disinfection cycle. The system is equipped with multi-level alarm, data storage traceability, permission management and remote monitoring functions. The electrical design complies with the local standards of Hazard Mara (120V/60Hz) and can adapt to grid fluctuations.
Technical advantages and performance features
The water quality of the produced water exceeds the standards of the pharmacopoeia: The water produced by the system can stably meet the requirements for purified water and water for injection stipulated in pharmacopoeias such as USP, EP, and ChP. Key indicators include Bacterial endotoxin ≤0.001 EU/mL (if WFI is produced), microbial ≤1 CFU/100 ml, total organic carbon (TOC) ≤50 ppb, resistivity ≥10 MΩ·cm (at 25℃).
Ultimate material guarantee and long-term reliability: The full set of SS316L material fundamentally eliminates the risks of metal ion precipitation and corrosion pollution that may be brought by low-grade materials. The equipment has a long service life and low maintenance costs, and is especially suitable for pharmaceutical projects with extreme requirements for water purity and system durability.
High degree of automation and compliance: The system design complies with GMP principles and is equipped with complete validation support documents (DQ/IQ/OQ/PQ). Fully automatic operation significantly reduces the operational burden and the risk of human error, and the audit trail function meets the data integrity requirements of the pharmaceutical industry.
Strong environmental adaptability: Specifically designed for heat dissipation, moisture-proofing and anti-corrosion in the tropical climates of Guatemala and Central America, it ensures stable performance of the equipment in high-temperature and high-humidity environments, continuously providing safe and reliable pharmaceutic-grade pure water.
