In lab daily operations, water purity is critical to experiment success. Google searches often ask: “Should labs use deionized or distilled water?” and “What if I use the wrong one?” Countless researchers are confused—different experiments (e.g., chemical analysis, microbial culture) need varying water purity. Wrong water causes bad data, equipment damage, or total failure. This article explores their lab differences to help you choose right.
Laboratory Deionized Water
Deionized water, commonly referred to by the abbreviation “DI water” in labs is the preferred choice for a variety of applications due to its efficacy in removing Ionic impurities.
Preparation Process
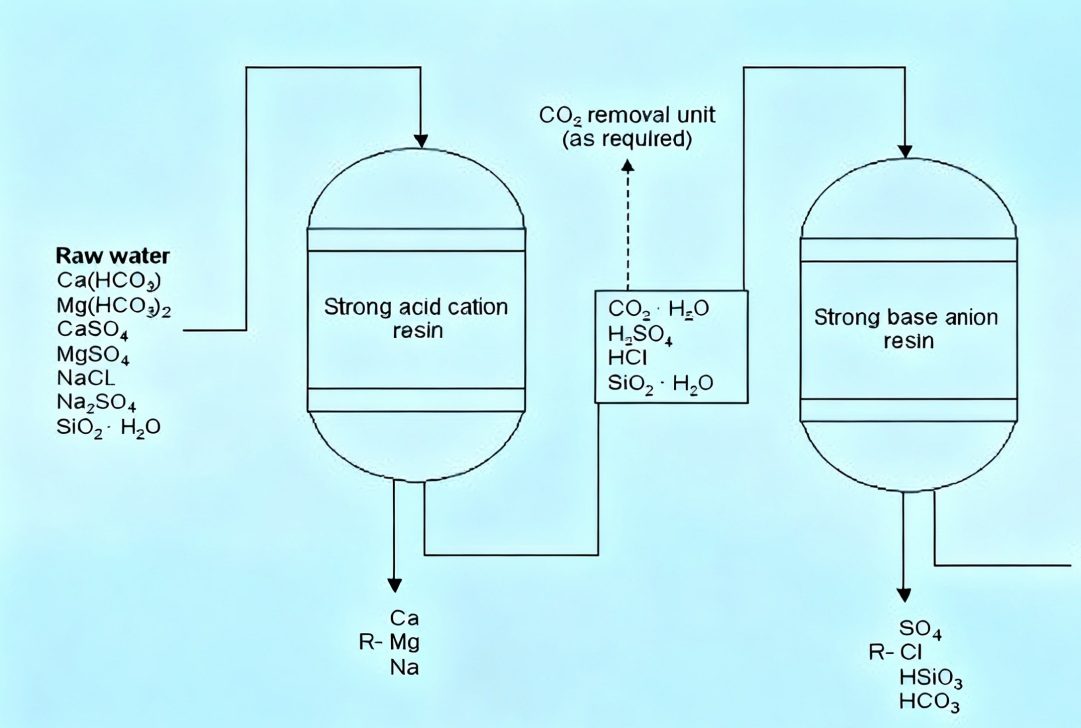
The purification of lab water deionized is primarily dependent on Ion exchange resins. Raw water, including tap water, has a variety of charged ions such as calcium (Ca²⁺) and magnesium(Mg²⁺) along with chlorine (Cl⁻). When water flows through ion exchange resins the resins function in the role of “ion trappers”: cation exchange resins swap positively charged ions in water with hydrogen Ions (H⁺) as anion exchange resins exchange negatively charged ions by using hydrogen ions (OH⁻). The H⁺ and OH⁻ ions join to make purified water molecules drastically diminishing the presence of ionic contaminants that are present in water. Certain advanced deionized water systems also incorporate reverse the process of osmosis (RO) pre-treatment to capture macromolecular impurities as well as improve the quality of water.
Key Characteristic
In lab settings, resistance (measured in MΩ·cm) is the most important indicator of the deionized water’s purity. For simple tests deionized water with resistances of 1-5 MΩ·cm is sufficient. However tests that are extremely susceptible to interference from ions, such as HPLC analysis which is a high performance analysis using liquid (HPLC) study, requires ultra-pure, pure water that has resistance within the range of >=18.2 MΩ·cm. It should be noted that even though deionized water is “zero-tolerance” for ions, it’s not effective against non-ionic contaminants including bacteria, viruses, as well as organic molecules. Other methods (e.g. UV disinfection or filtration) are necessary in the situation where your test may be sensitive microorganisms, organics, or other substances.
Laboratory Distilled Water
Distilled water, a classic yet reliable purified water kind is highly valued due to its purity and exceptional quality, making it a must for laboratory applications.
Preparation Process
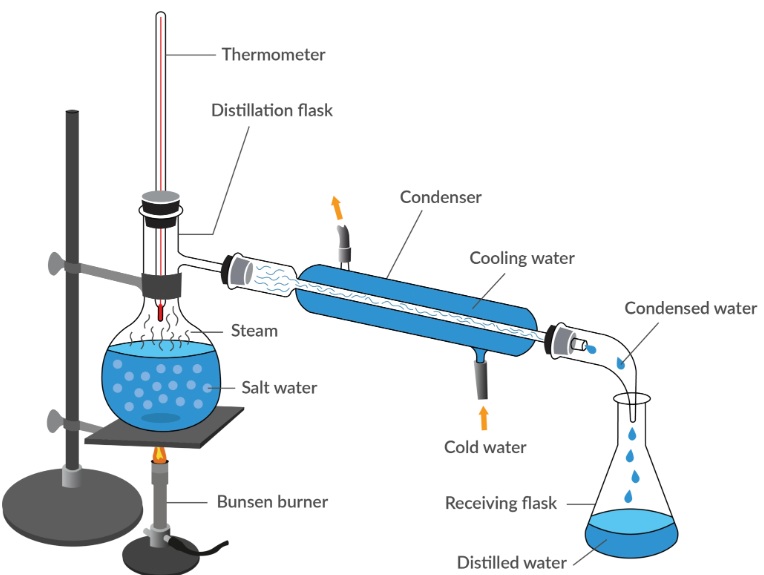
Laboratory-produced distilled water is made using the standard procedure that involves “heating to vaporization + condensation and collection”. The water is heated up to 100 °C (boiling points) to produce steam and impurities that have more boiling point than water remain in the container that originally contained it. The steam is then cool by a condenser before being it is condensed into liquid water that is then collected as distillate water. This process functions as an “purity grading system” for water, and removes the majority of impurities like the microorganisms and ions as well as organic pollutants and particles, which results in the purest water.
Key Characteristic
Distilled water is extremely low conductivity (high-quality distillate water may be as low as <0.5 μS/cm) and the microbial count is of 1 CFU/mL, in full compliance with the standards for laboratory grade 1 water, or even higher standards. In experiments requiring near-perfect purity-such as active ingredient analysis in pharmaceutical research or sample preparation for high-end electron microscopes-distilled water is the unrivaled choice due to its unparalleled purity. But the distillation process requires a lot of energy and takes time and results in a inefficient water production.
Essential Differences in Laboratory Scenarios
You need to know the core difference between deionized water or distilled water can be essential to make informed decisions in laboratory conditions.
Production Efficiency and Cost
| Comparison Factor | Laboratory Deionized Water | Laboratory Distilled Water |
| Production Efficiency | The equipment is quick to start and allows continuous water production, which is ideal for large-scale, high-frequency requirements. | Long production cycles, with only a small amount of batch output. It requires condenser and heating cycles, which is not suitable for situations that require instantaneous huge quantities of water. |
| Cost Investment | A lower initial investment in equipment Ion exchange resins, however, require periodic replacement or renewal (long-term consumable expenses, e.g., resin renewal every 3-6 months, based on the water consumption). | Cost of distillation equipment is higher, due to the high cost of purchasing equipment and a high consumption of energy in operation, however no regular replacement of consumables is required. |
Composition and Safety
Deionized water: low Ion content, however it may retain organic molecules and microorganisms. In the microbial culture experiment, leftover microorganisms battle with targets for nutrients, which can lead to problems with the culture. Organic molecules can disrupt the sensitive chemical analysis and cause data errors. In cell-based experiments residues of impurities could impact the growth of cells.
Distilled Water Distilled Water: Distilled Water “zero impurities” (significant reduction in organics, microorganisms and even ions) and high-security. However, during distillation volatile organic contaminants (e.g. the benzene, formaldehyde) in the water could evaporate and then contaminate the final distillate. So, certain high-end distillation systems have an activated carbon filtering step to further cleanse the water.
Choosing the Right Water for Different Laboratory Experiments
The selection of the right type of water is based on the specific needs for each experiment.
Chemical Analysis Experiments
Ionic interference is considered to be the “number one enemy” in chemical analysis.
Application-specific scenarios: In the titration process, ions present are present in the water and react to titrants which can interfere with the endpoint determination. In the atomic absorption spectroscopy (AAS) and Ion Chromatography (IC) studies, ionic impurities could cause false positive signals that can seriously impairing detection accuracy.
Recommended Choice: Deionized Water (resistivity greater than 10 MΩ·cm). For instance, in acid-base titrations, the use of high-purity deionized water makes sure that the variations in pH are just influenced by the titration process which avoids the errors that can be caused by interference from ionic.
Not recommended: Normal distillate water (without the ability to effectively remove volatile organics) can affect the base of chromatographic analyses which could affect data stability unless the test has no requirement in organic impurities.

Microbial and Biological Experiments
It is also an essential “lifeline” for microbial and biological research.
Possible Scenarios: In microbial cultivation it is possible for a tiny amount of contaminating bacteria could take over the dish and grow which leaves no room for microorganisms targeted by. In PCR experiments, nucleic acids fragments from microorganisms present in water interfere with the results of amplification and can cause fake positive band.
Most preferred option: Distilled water (after an autoclave or 0.22 μm membrane filtration). For cell culture experiments only organic-free, sterile distillate water can provide the right environment to promote cell growth, which will ensure the survival of healthy cells and their proliferation.
Not Recommended: Non-sterilized deionized water–microorganisms and organic pollutants can severely affect experiments and lead to failure.
Equipment Usage
The protection of precision instruments is essential to ensuring the efficiency of labs and reducing the cost of maintenance.
Possible Scenarios: HPLC preparatory phase mobile particles or ions within water can block the chromatographic columns, reducing their useful life. For the atomic fluorescence analysis (AFS) carriers, water that is not of high quality affects the efficiency of atomization as well as reduces the sensitivity of the detection. Autoclaves and lab washers prolonged use of unclean water causes scaling, which can damage heating tubes.
Preferred Choice: Instruments that are precision (e.g., HPLC, AFS) Deionized water (resistivity >=18.2 MΩ·cm with 0.22 μm membrane filtration) to ensure that no particles or ions hinder the operation of the instrument. Autoclaves, washers, and other equipment: Deionized Water (resistivity greater than 1 MΩ·cm) to avoid the build-up of scale and extend the lifespan of the equipment.
Not Recommended: Tap water or deionized water with low purity – impurities can easily cause damage to equipment and raise the cost of maintenance.

Common Misconceptions About Laboratory Water
Clearing up myths helps you avoid mistakes caused by using the wrong type of water.
Myth 1: “The Higher the Purity of Deionized Water, the Better”
Correction: Different experiments have different standards for purity. For regular glassware cleaning Grade 3 deionized water (resistivity >=0.2 MΩ·cm) is enough. HPLC analysis however calls for Grade 1, (resistivity >=18.2 MΩ·cm). In blindly using water with high purity, it not only adds costs, but creates problems: water with high purity quickly absorbs carbon dioxide from air, decreasing its pH and hindering studies that require pH (e.g. acid-base neutralization Titration).
Myth 2: “Distilled Water and Deionized Water Can Be Used Interchangeably”
Correction: In microbial studies deionized water can’t substitute for distillate water (due to the residual microorganisms). For large-volume chemical analyses the that of deionized waters (due to its low efficiency in production). For example, deionized water is more efficient for daily large-volume reagent preparation, while experiments with strict restrictions on microorganisms/organics (e.g., cytotoxicity testing)
Misconception 3: “Laboratory Water Can Be Stored for Long-Term Use After Preparation”
Correction: Water that is deionized for longer than 24 hours shows an increase in resistance (due to the fact that it absorbs Ions from in the atmosphere). Distilled water that is stored for longer than 7 days could develop microorganisms. It is advised to drink the water right away after preparing. If storage is required make sure you use sealed containers, sterile polyethylene containers, and store them at 4°C Re-test the quality of water prior to using.
Conclusion
Selecting the appropriate water type is the first step to getting accurate results from experiments and protecting equipment in the lab and increasing the efficiency of research. The laboratory deionized water is a leader in removing ionic impurities as well as production efficiency, which makes it the ideal choice for general chemical analysis and maintenance of instruments. Water from laboratories, which is distilled to its high quality, is essential for microbial culture, delicate biological experiments, as well as high-precision analysis.
Need a Reliable Lab Deionized Water System? We provide comprehensive, tailored solutions to meet every lab’s purity requirement, our flagship product series, including Molgene, Molelement, and Molcel etc.Visit Molewater today and find the ideal water purification solution for your lab.






