In industries with extremely high Water quality requirements such as pharmaceuticals, healthcare, and biotechnology, many people often encounter the same question when choosing water: What Exactly is the Difference Between WFI And Purified Water?
They all seem very pure, are all used for the processing of medicines or equipment, and sometimes even come from the same system. However, due to different uses and standards, the prices vary significantly, and the risks of use are also vastly different. Many users may use the wrong water without understanding the specific differences. This not only leads to non-compliance of the products but also may cause quality defects or even safety accidents.
This blog is precisely designed to address this common confusion, fundamentally explaining the definitions, processes, standards, applications, and key points for selection of the two types of water, helping you make correct judgments and avoid unnecessary risks caused by incorrect water usage.

The Definitions Of WFI And Purified Water Are Different
To understand the essential difference between WFI and purified water, the first step is to know their definitions and sources.
Purified Water
Purified water refers to water that has undergone a series of physical or chemical methods (such as reverse osmosis, ion exchange, electro-deionization, distillation, etc.) to remove the vast majority of soluble ions, organic matter, particulate impurities and microorganisms. Its water purity is relatively high, but strictly speaking, it may still contain trace amounts of pyrogens or extremely small amounts of microorganisms, and is not suitable for injection purposes that require complete sterility and pyrogen-free treatment.
Its applicable standards are derived from pharmacopoeias of various countries, such as USP, EP, JP, etc., and mainly focus on the following indicators:
- Electrical conductivity
- Total Organic Carbon (TOC)
- Microbial limit

WFI (Water for Injection)
WFI is further processed on the basis of purified water. It usually adopts multi-effect distillation or ultrafiltration technology that meets the requirements of the pharmacopoeia to remove all contaminants that may harm the human body, especially pyrogens (endotoxins) and microorganisms, to achieve sterility. WFI is one of the purest waters in pharmaceutical production.
The applicable standards are stricter, covering not only all the indicators of purified water but also adding:
- Pyrogen control (endotoxin limit shall not exceed 0.25 EU/mL)
- Microbial limit control is stricter
- It is generally required to be made and used immediately or within the specified time
Key Differences Between WFI and Purified Water
Let’s compare the specific differences between WFI and purified water item by item from several key dimensions:
| Comparison Aspect | Purified Water | Water for Injection (WFI) |
| Definition & Source | Water purified by methods like RO, distillation, or ion exchange | Further processed purified water, typically by distillation or ultrafiltration |
| Main Usage | General pharmaceutical uses: cleaning, non-injectable formulations, solution prep | Injectable drug preparation, final rinse for injectable containers, aseptic processes |
| Microbial Control | Microbial limit typically ≤ 100 CFU/mL (per pharmacopeia) | Much stricter microbial limit; often required to be sterile |
| Endotoxin Requirement | Not required to test for endotoxins | Mandatory endotoxin control (≤ 0.25 EU/mL) |
| Production Process | Flexible: RO, EDI, distillation, etc. | Must use distillation or validated membrane-based methods |
| Storage Requirements | Can be stored in controlled loop systems | Typically stored hot (> 80°C) or used immediately to prevent contamination |
| Cost and Risk | Lower cost, easier to produce, suitable for broader use | Higher cost, regulatory risk if misused, essential for patient safety in injectables |
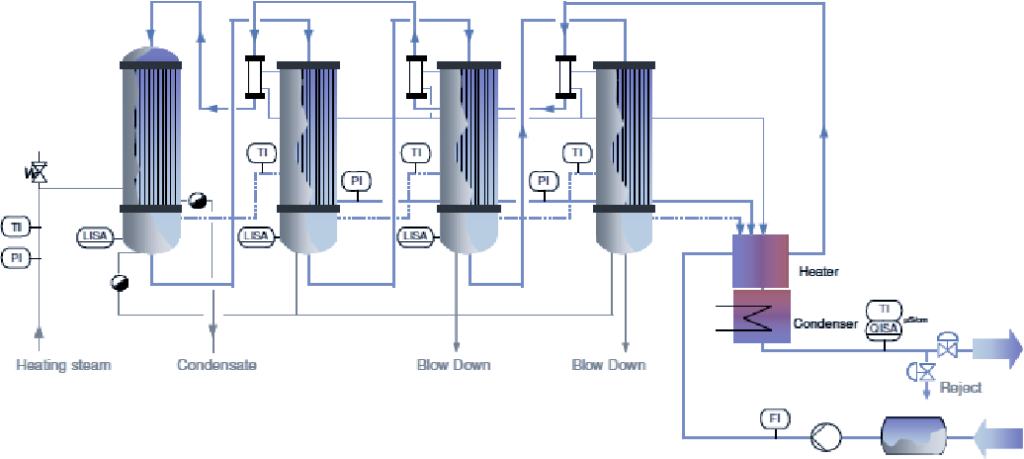
How To Choose The Right Water?
The quality requirements for water in different pharmaceutical production processes vary. The following are typical application differences:
➤ Application scenarios of purified water:
- Production of non-injectable drugs (such as tablets, oral liquids, and topical preparations)
- Dissolution and mixing of raw and auxiliary materials
- Water for laboratory analysis
- Intermediate cleaning of equipment, containers and pipelines
- The basic water used in certain cosmetics or food processing
➤ Application scenarios of WFI:
- Solvents for injections, such as water injections, infusions, vaccines, etc
- Preparation of sterile active pharmaceutical ingredients (apis)
- Final cleaning of injection containers (ampoules, syringes, etc.)
- Bottle washing and final preparation in the filling process
- All links that come into contact with human blood, tissues or injection sites
A simple criterion for judgment is:
Whenever water eventually enters the human body (through injection, perfusion, etc.), WFI must be used. Otherwise, purified water can be selected based on the risk assessment.
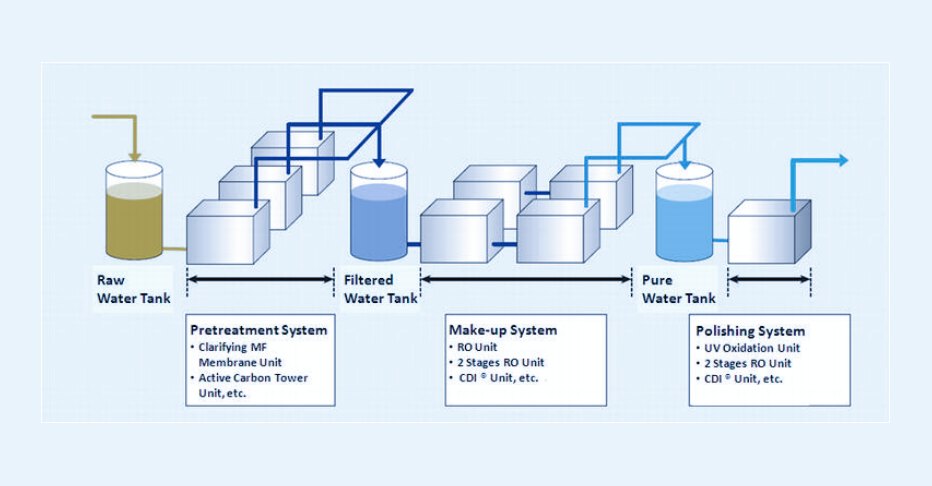
Suggestions For Choosing Water
The design of water systems and the selection of water usage in the pharmaceutical industry must not only comply with regulatory requirements but also conform to the principles of risk management. The following suggestions can help you make a more reasonable choice:
Identify key uses
Clarifying the end use of water is the first step in making a choice. As long as there is a direct or indirect risk of entering the human body, do not hesitate to choose WFI.
Don’t substitute price for quality
Using purified water instead of WFI may save costs in the short term, but once the product is non-compliant, microorganisms exceed the standard or pyrogenic reactions occur, the cost paid will be much higher than the initial savings.
Understand the differences in regulatory requirements
The definitions and testing standards for purified water and WFI in pharmacopoeias of various countries are slightly different. For instance, USP has accepted the cold method (membrane method) for the preparation of WFI, while the European Union still requires that WFI must be distilled. Special attention should be paid in actual use.
Ensure system verification and monitoring
Whether it is purified water or a WFI system, it must be verified (IQ/OQ/PQ) to ensure stable operation and continuously monitor key indicators such as TOC, conductivity, temperature and microorganisms.
Final Words
WFI and purified water may have no difference in appearance, but the preparation requirements, quality standards and application scenarios behind them are quite different. On the zero-tolerance risk front of the pharmaceutical industry, using the wrong water means product failure, regulatory violations, and even patient risks. Therefore, fully understanding the differences between them is a fundamental knowledge that every pharmaceutical, quality control, equipment or validation engineer must master. Don’t let a single drop of water become the starting point of quality risks. Making the right choice means adhering to the bottom line of compliance and is also the most fundamental respect for safety.
As a professional manufacturer and solution provider of pharmaceutical water systems, Molewater has long been dedicated to the design, manufacture and validation of purified water and injection water systems, helping users achieve higher standards of water safety and compliance.





