In the pharmaceutical industry, the goal of sterility isn’t only a requirement, but an essential element. A single microbial infestation in the form of an injectable drug or vaccine could cause grave patient harm as well as fines from regulatory authorities or recalls of products. The key to keeping this sterility in check is an essential component that is the pure steam generator pharmaceutical system. These special systems create pure steam that is free of mineral, endotoxins and microbes, which makes essential for sterilization processes. Let’s look at the ways in which pure steam generation system technology assures sterility, and why selecting the right clean steam generator manufacturers is important, and why Molewater is a reliable supplier.

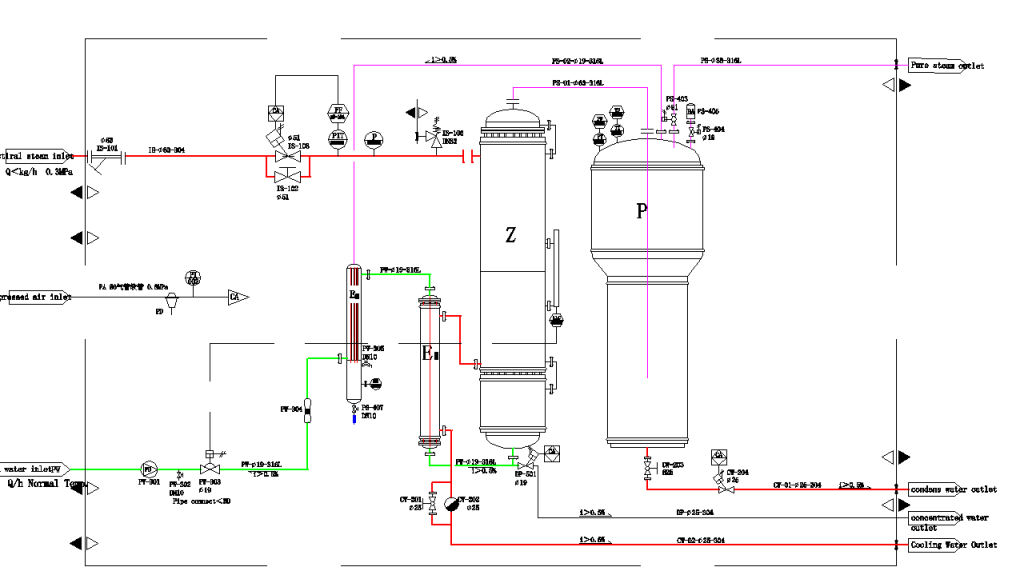
Pure steam generation systems transforms high-purity feedwater -typically the term is Water for Injection (WFI)-into steam via precision heat exchange. Contrary to industrial steam produced by boilers that may be contaminated with mineral residues, pharmaceutical grade steam systems are 100% pure and provide a clean output via distillation and separation.
At Molewater, our Pure Steam Generation System (PSG) uses industrial steam to heat purified water inside an evaporator made of shell and tube. The steam is then separated into pure steam, and then checked online to ensure consistency in quality and pressure.
Pure Steam Generators Ensure Sterility in Pharmaceutical Processes
Pure steam generator pharmaceutical systems ensure sterility through precise engineering and rigorous purification.
Removal of contaminants
The injectables in pharmaceuticals are unable to accept even trace amounts of impurities. Pure steam generators remove pollutants through a multi-stage purification process. In distillation, mineral salts (like magnesium and calcium) as well as heavy metals and endotoxins (harmful bacteria byproducts) remain in the liquid residue since their boiling point is higher than that of water. The resultant steam is clean of dissolved solids, pyrogens, as well as particulate matter.
Microbial eradication
Microbes, including viruses, bacteria and their spores-are the main danger to pharmaceuticals that are sterile. Pure steam neutralizes pathogens by destroying them with heat: under pressure that is controlled it reaches temperatures of 121 to 134 degrees Celsius, which is far beyond the range that is required to denature microbial protein and degrade their cellular structures.
This thermal sterilization process is superior over chemicals (like hydrogen peroxide and ethylene oxide) because of a number of reasons. Chemicals leave harmful residues on products or equipment contact surfaces, and require intensive rinsing to avoid contamination. Pure steam, however is free of residue and only harmless condensate. This makes it perfect to sterilize items that have in direct contact with drugs, like vials, syringes, or filling needles.
Uniform sterilization
Sterilization can only be effective if each surface and crevice is heated to a sufficient degree. Pure steam generators tackle this issue by ensuring precise control of the dryness of steam and its pressure. This ensures “saturated dry steam” reaches even the most difficult-to-access regions.
Dry steam that is saturated (with a dryness ratio of 0.95-1.0) is the most efficient source of thermal energy, which allows it to condense over cool areas and then transfer heat evenly. This helps to prevent “cold spots”-areas in which temperatures are below the sterilization threshold – where bacteria can thrive. Furthermore, steam systems that are pure are designed to limit the condensability of gases that are not condensable, which can act as insulation on surfaces and hinder heat transfer.
Applications of Pure Steam Generators in Sterile Pharmaceutical Production
Pure steam generators can be employed extensively in sterile manufacturing processes:
Equipment Sterilization
Sterilization-in-Place (SIP) processes depend on pure steam to thoroughly sterilize reactors, holding tanks, transfer pipelines, autoclaves, and vial-filling systems. Molewater’s systems offer reliable temperature steam (121–134°C), which ensures the destruction of all bacteria, spores, and viruses. The saturated and dry steam technology guarantees uniform sterilization, without condensate pooling, which is a danger to the growth of microbial species.
Cleanroom Sanitization
Cleanrooms of Grade A/B (where injections of injectables take place) require a strict control of microbial growth (<=10 CFU/m3 in Zones of Grade A). Pure steam is invaluable here, as it can sanitize surfaces, walls, and air handlers in ways chemical disinfectants cannot.
Unlike wipes or sprays, which may miss cracks or high-touch areas, steam can reach into crevices, under equipment, and around fixtures, killing microbes on contact. Molewater’s systems support both surface steaming and fogging applications, where steam is atomized into fine droplets to sanitize air and hard-to-reach spaces. This leaves no chemical residues, reducing the risk of product contamination and simplifying cleaning validation.
Injectable and Biologic Production
In the production of injectables and biologics every touchpoint—from vials and stoppers to filling needles and isolators—must be sterile. Pure steam is used to sterilize these components, either in autoclavesor directly in aseptic processing lines .
For example, vaccine manufacturing relies on pure steam to sterilize freeze-dryer chambers and vial capping machines, ensuring no microbes are introduced during the critical final stages of production. In biologic manufacturing, where even trace endotoxins can trigger severe immune reactions, pure steam’s ability to eliminate pyrogens is especially critical.
Choosing the Right Pure Steam Generator Manufacturers
The selection of a reliable pure steam generator manufacturer is crucial in ensuring sterility assurance in the pharmaceutical production. Be aware of the following elements: reliable manufacturers provide complete validation tools (IQ/OQ/PQ protocols, material traceability) and adhere to GMP, FDA 21 CFR Part 11, and EU Pharmacopoeia standards-for example, uses 316L stainless steel and ASME BPE sanitary design to control endotoxins and bioburden.
Scalability matters—top manufacturers offer diverse outputs, like Molewater’s PSG 100-600 with modular skids for easy expansion. Purity is non-negotiable, meeting WFI standards (conductivity ≤1.3 µS/cm, etc.), ensured auto blow-down, and double-tube evaporators.
Molewater: Your Partner for Pharmaceutical Pure Steam Generator
Pure steam generators are not just equipment—they are guardians of sterility in pharmaceutical manufacturing. By removing contaminants, eradicating microbes, and ensuring uniform sterilization, they play a pivotal role in protecting patients and maintaining regulatory compliance. From SIP processes to cleanroom sanitization and biologic production, their applications are integral to every stage of sterile drug manufacturing.

For pharmaceutical companies, investing in a reliable pure steam generator is an investment in safety. Molewater’s PSG series, with its precision engineering, real-time monitoring, and compliance with global standards, stands as a trusted partner in this mission. To learn how our systems can elevate your sterile manufacturing processes, contact Molewater today.





