In pharmaceutical production, where tiny impurities risk drug safety and compliance, water quality is non-negotiable. Yet many facilities miss hard water’s threat: its calcium/magnesium ions cause sterilizer scale, filter clogs, injectable precipitates, delayed GMP re-evaluations, and costly batch scrapping. Why is hard water critical for pharma? How to soften it reliably without losing compliance? This article answers these, focusing on pharmaceutical-grade ion exchange resins—explaining how the ion exchange resin water softener transforms hard water, unique advantages, and why they safeguard production.
Why Hard Water Is A Problem?
Pharmaceutical production requires more stringent standard of water than most other fields. Good Manufacturing Practice (GMP) establishes strict standards for quality and hardness of water (e.g. for injectable and oral preparations) However the minerals calcium, magnesium and iron present in hard water do not conform to GMP standards, which poses three significant risk factors:
Threats to Product Quality & Medication Safety
Metal ions within hard water can react to components of liquid medicine, which can result in the liquid medicine components to become unstable. For instance the calcium ions resulting from injections can result in calcium precipitates insoluble that could lead to vascular embolisms when they are are administered. Iron ions are able to oxidize active ingredients in oral liquids (tablets/capsules) and result in discoloration, less substance and a reduction in efficacy. Impurities can make products ineffective “visible foreign matter” or “purity” tests, leading to the necessity of the reworking or scrapping—issues the ion exchange resin water softener is designed to resolve.
Faster Equipment Wear & Higher Failure Risks
Precision equipment (distillers, sterilization cabinets) operates under high temperature/pressure. 1mm scale on sterilization cabinets extends heating time by 20% (increasing energy use and destabilizing sterilization). Scale gaps breed hard-to-remove microbes, failing cleaning verification and risking cross-contamination. Hard water also clogs filters/pipelines, interrupting purified water supply and halting production.
Higher Costs & Compliance Issues
Hard water causes “explicit costs” (filter replacement, equipment cleaning, scrapped medicines) and “implicit costs”: failed water tests force production halts, delaying GMP certification or product registration. One biopharma firm’s innovative drug launch was delayed 3 months due to hard purified water, missing market opportunities.
Ion Exchange Resin Technology:A reliable Answer
To address hard water hazards, pharma firms need softening solutions that meet both precision and compliance standards. Pharmaceutical-grade ion exchange resins stand out—they remove hard water metal ions accurately and fit strict production demands via compliant materials and stable performance, making the ion exchange resin water softener a trusted choice.
How Ion Exchange Resins Soften Water in Pharma Scenarios
Unlike industrial resins, pharmaceutical-grade ones meet FDA/EP standards and pass “low extractable” tests to avoid harmful releases. Two common types exist:
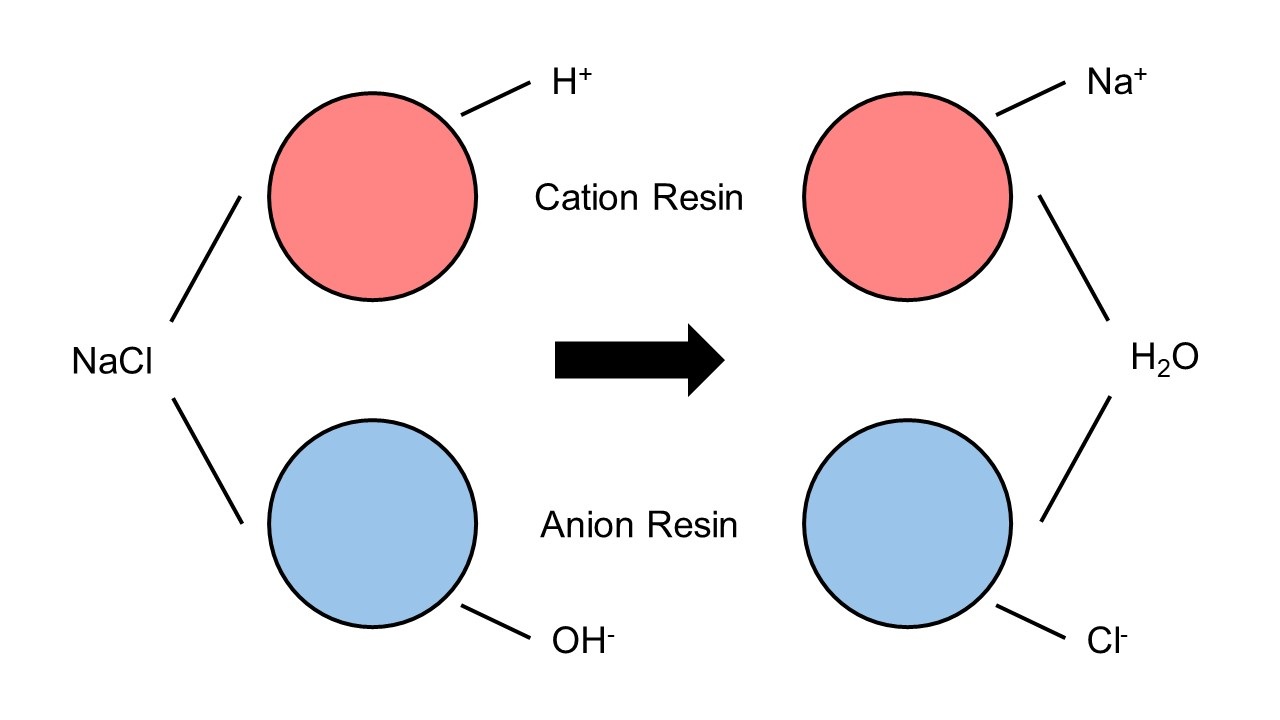
Sodium-based cation resins: For softened water (e.g., oral prep cleaning). Sodium ions in resins exchange with hard water’s calcium/magnesium, adsorbing the latter and outputting water with hardness <0.03 mmol/L.
Mixed-bed resins: Blend cation and anion resins for purified/injection water. They remove calcium/magnesium and anions (chloride, sulfate), achieving 18.2 MΩ・cm resistivity (meets injection water standards).
The process uses no chemicals—only physical exchange. Resins are reusable via regeneration: rinsing with pharmaceutical-grade sodium chloride/hydrochloric acid displaces adsorbed ions, restoring capacity and preventing secondary pollution.
Three Core Advantages
(1) High Precision for Multiple Scenarios
Oral prep cleaning needs hardness <0.1 mmol/L; injection water needs near-zero hardness. The ion exchange resin water softener adjusts via type (single/mixed-bed) and parameters—sodium-based resins lower hardness to <0.03 mmol/L, while mixed-bed resins produce ultrapure water, covering all production stages.
(2) Strong Compliance & Traceability
Resins meet GMP from selection to use. Suppliers provide COA/DMF documents; use data (regenerants, hardness tests, replacement cycles) is traceable in “water quality files,” aiding audits. One vaccine firm passed GMP re-evaluation once with compliant resins.
(3) Stable for 24/7 Production
Resins have high exchange capacity (stable for weeks) and support double/multi-column designs—one column regenerates while the other supplies water, avoiding interruptions. An infusion firm’s double-column system achieved 99.8% annual supply stability.
What Sets Ion Exchange Apart?
In the field of softening hard water in the pharmaceutical industry in addition to Ion exchange resins different methods like electrodialysis and reverse-osmosis are also available. But, they differ in regards to price flexibility, adaptability, and compliance, that is why companies should select according to their particular requirements. Three technologies are described below in relation to four points, which include precision in treatment as well as compliance expenses and their adaptability to a variety of circumstances within the industry of pharmaceuticals.
| Dimension | Ion Exchange Resins | Electrodialysis | Reverse Osmosis |
| Treatment Precision | High (removes trace ions, suitable for purified/injection water) | Medium (removes partial Ca²⁺/Mg²⁺, for raw water pretreatment) | High (removes most ions, but sensitive to inlet water hardness) |
| Compliance | High (pharmaceutical-grade, provides DMF/COA documents) | Medium (membrane leachables need extra testing, weak traceability) | Medium (membranes meet pharmaceutical standards, but replacement records are easy to miss) |
| Operating Cost | Medium (regenerable, ~hundreds of yuan per regeneration) | Low (low equipment cost, high energy consumption, membrane life 1–2 years) | High (high initial investment, ~tens of thousands of yuan per membrane replacement) |
| Pharma Adaptability | Wide (for cleaning, liquid preparation, injection water) | Narrow (only for pretreatment, unable to meet purified water needs) | Specific (needs pretreatment to avoid scaling, for high-purity water) |
Ion Exchange Resins excel in “balance”: it matches reverse osmosis in precision while controlling cost, and has electrodialysis’ flexibility while meeting compliance. For enterprises needing full-process water coverage (e.g., producing both oral and injectable preparations), it adapts to all links via single/mixed beds, no need for multiple systems, offering higher cost-effectiveness.
Why Choose Ion Exchange For Hard Water To Soft Water Treatment?
Beyond technical perks, ion exchange resins water softener solve practical “implementation challenges” in pharmaceutical hard water softening—making them the mainstream choice:
Strong Water Quality Control for Multi-Dosage Production
Pharma firms often make multiple dosage forms (tablets, injections, oral liquids) with varying water hardness needs. Ion exchange resins enable flexible switching via “resin combination adjustment”: sodium-based cation resins for oral tablet water, and added mixed-bed resins for injection water—no full system replacement. One enterprise cut water quality adjustment time from 4 hours to 30 minutes, boosting flexibility.
Controllable Costs, Lower Lifecycle Investment
Cost-sensitive pharma firms benefit from resins’ “regenerable” trait, which cuts long-term maintenance costs. Versus reverse osmosis, resin systems have moderate initial investment. Resins are regenerated multiple times with low-cost pharmaceutical-grade agents and last longer, leading to far lower total lifecycle costs than reverse osmosis (which needs costly regular membrane replacements).
Good Equipment Compatibility, No Major Modifications
Most firms already have mature purified/injection water systems. New tech may require costly, production-disrupting equipment removal, but compact, flexible resin systems connect directly to existing setups: add a mixed-bed column post-purifier for better precision, or a sodium-based column pre-cleaning pipeline for softening. One TCM firm finished installation/commissioning in 3 days, with no production impact.
Avoid Heavy Metal Risks, Ensure Drug Safety
Pharmaceutical raw water may have lead, mercury, cadmium (from pipe corrosion/pollution)—ions unremovable by regular filtration but harmful to drugs. Pharma-grade resins (especially chelating types) adsorb 99.9% of heavy metals. A children’s drug firm used them to keep drug heavy metal content below 0.001mg/kg, well under the 0.005mg/kg pharmacopoeia standard.
Final Thoughts
In pharmaceutical production, water quality is an invisible lifeline, yet hard water—like a hidden reef—quietly threatens it. It damages drug purity, creates medication safety risks, accelerates wear of precision equipment (e.g., sterilization cabinets, liquid tanks), and even blocks compliance due to substandard water, directly endangering business survival.
Compared with electrodialysis (limited precision) and reverse osmosis (high cost), pharmaceutical-grade ion exchange resins, with high water precision, strong compliance and controllable long-term costs, are ideal for pharmaceutical hard water softening. Need a one-stop solution? Choose Molewater’s professional Industrial Water Softener to secure your pharmaceutical production water.