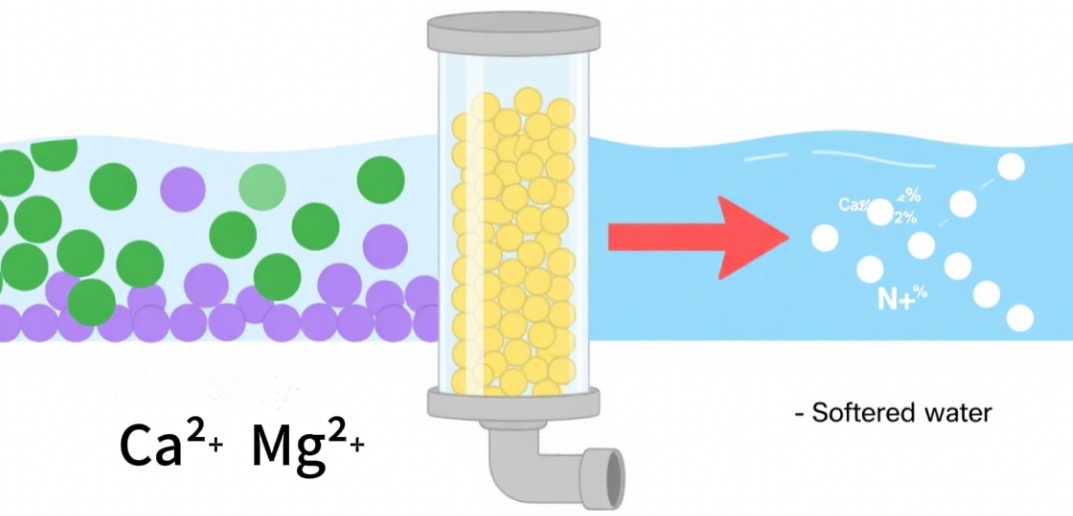
Hard water, which is rich in calcium (Ca²⁺) and magnesium (Mg²⁺) ions, creates frustrations like limescale accumulation in pipes, decreased effectiveness of water heaters, along with dull laundry. To solve these issues, ion exchange water softeners are regarded as a reliable and widely-used solution. But how does the ion exchange process work in water treatment? What is it that makes the process of ion exchange efficient to treat water? This article explains the working principles, science behind it and the real-world applications of the technology.

The Ion Exchange Process
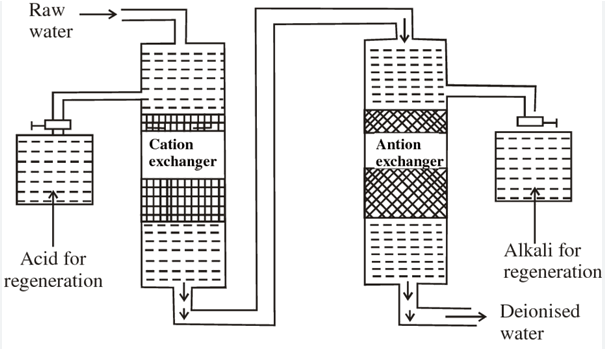
In its essence ion exchange is a chemical-physical process that makes use of an “ion exchanger” (like resins or Zeolites) to swap the ions in a solution, here water. Its efficacy is due to three main characteristics:
- Reversibility: The exchange reaction is able to be reversed using regenerant (e.g., NaCl for Cation resins). Ion exchangers can be recyclable, making the process economically viable over time.
- Selectivity: Ion exchangers have a preference for certain Ions over other ions. For instance, cation-based resins prefer the ions with high charges (e.g., Fe³⁺ > Ca²⁺ > Na⁺) due to the fact that stronger electrical attractors are more able to bind them.
- Quantity: Every Ion exchanger has an fixed “exchange capacity”, which is the amount of ions it is able to exchange in order to achieve adsorption. This is done by chemical stoichiometry, which ensures an even performance when properly operated.
This process involves three fundamental elements: the Ion exchanger (the reacting medium) as well as the water that is to be treated (containing the ions that are targeted, such as Ca²⁺ or Cl⁻) and an optional regenerator (to replenish the exchanger).
The Principle of Ion Exchange
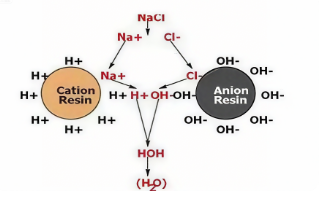
Ion exchange is based on two primary types of resins: Cation exchange resins as well as anion exchange resins. Each one targets specific ions and is based on different fundamentals.
1. Cation Exchange Resins
Cation resins are cross-linked polystyrene matrices with functional groups with acidic surfaces that determine their performance.
In addition, highly acidic resins are sulfuric acid group (-SO₃H) which function in neutral, acidic as well as alkaline waters. They release Na+ or H+ to exchange with water cations, such as Mg²⁺, Ca²⁺, and Fe³⁺.
The weakly acidic resins have carbonoxyl group (-COOH) which only function in neutral or alkaline water. They are also more effective at removing divalentions (e.g., Ca²⁺) and require less regenerating agents and have a lower cost of production.
Their selection is based on a rule that binds cations with higher charges first (Fe³⁺>Ca²⁺>Na⁺). For cations with the same charge, a larger Ionic radius increases binding strength (Ca²⁺>Mg²⁺).
2. Anion Exchange Resins
Anion resins have the same matrix of polystyrene as Cation resins, but have basic functional groups and they are able to target negatively charged anion (anions) like Cl⁻, SO₄²⁻and NO₃⁻.
Strongly basic resins have Quaternary Ammonium Groups (-N(CH₃)₃OH) and function at all levels of pH. In the course of ion exchange they release hydroxide ions (OH⁻) and the chloride Ions (Cl⁻)and exchange them with toxic anions within water.
The weakly basic resins contain secondary (-NH₂) as well as second (-NHR) amino-groups and only work in water that is acidic. They are particularly effective in getting rid of organic acids and are much easier to regenerate when compared to resins that are strongly basic.
The anion resin’s selectiveness is comparable to that of cation-based resins. The ions with the highest charge are preferred by a standard order of SO₄²⁻ > HCO₃⁻ > Cl⁻. For anions that have similar charges less hydrophilic, they are bound more tightly for example, NO₃⁻which are more binding than Cl⁻.
The Ion Exchange Process in Water Treatment Works
Beyond softening, ion exchange can be a water treatment tool that is versatile that works by following five main steps.
A pre–treatment acts as the initial protection. Raw water is filtered and settled or passed through multi-media filters to eliminate the suspended particles, colloids and organic matter from the environment prior to them reaching the resin. This ensures the resin’s porosity and helps prevent irreparable contamination that may require costly cleaning up of chemicals later on.
The next step comes the ion-exchange phase. Most plants use fixed-bed columns. In these, water is pushed downwards through the stationary resin beads at 10–30 m h⁻¹, which is slow enough to permit diffusion, yet quick enough to meet demands. For small spaces or heavy loads fluidized beds are utilized where resin grains move upwards which exposes more surface and halving the column’s height. Operators keep track of pressure drop and bed expansion in order to maintain the area of mass transfer within the vessel.
On-line analyser systems keep track of every drop throughout operation. Conductivity probes and the hardness kits are connected to a PLC and, when the ion levels are higher than that set point, the system shifts to regeneration, and polished water inside the pipe is directed to tanks for finished water to prevent the waste.
Regeneration, a recurring crucial step, begins with a an aggressive back-wash that lifts the bed, get rid of particles of lint, broken grains and iron that has precipitated. After drained brine (or caustic in the case of anion-based units) is fed at a controlled intensity and speed to strip of ions that have been captured and to restore the resin’s active structure. A slow rinse eliminates any remaining regenerant. It is followed by a quick rinse until the effluent is in line with the water standards of the plant.
In the end, in standby mode, the control system records throughput, chemical usage and the bead’s pressure drop. Resins remain inflated and ready for the next cycle when the duty valve is opened.n of air, especially in RO systems that are salt-free, to avoid the growth of mold and the overheating of control panels.
Applications of The Ion Exchange Process in Water Treatment
Ion exchange’s versatility makes it an essential tool across different industries:
Water softener for industrial boilers
Industrial boilers require extremely soft water for their feed; even trace magnesium or calcium precipitates to form hard and tenacious limescales, which can block heat transfer surfaces, and causing local overheating or a catastrophic failure. Strong-acid cation resins made of sodium switch these hardness ions into safe sodium, providing water that is less than 0.1 per milligram CaCO₃ and ensures that boilers run in a safe manner at their maximum thermal efficiency.

Purification of drinking water
In the purification of drinking water, ion exchange is crucial roles. The cation resins with strong acidity focus on and eliminate harmful ions, such as fluoride (F⁻) and Nitrate (NO₃⁻)) as well as the anion resins that are weakly base get rid of remaining chlorine (Cl⁻). Furthermore, the softening effect of ion exchange decreases water hardness and greatly improves the taste of drinking water.
Ultrapure water for electronic devices
In the case of ultrapure water used in semiconductor manufacturing, electronics have specific requirements. It requires water with close to zero conductivity and ions, with a minimum of 0.1 μS/cm. This is accomplished through a mixture of anion resins, cation reagents as well as “mixed beds” (blended resins) in the treatment.
Treatment of wastewater and recovery of resources
Chelating cation resins are able to selectively take copper, nickel and chromium ions in complex industrial flows, and hold them until a tiny acid strip is returned to the metals that is pure enough to reuse or selling. Additionally strong-base anion resins pull harmful cyanide from the electroplating rinse water, and strip sulfate out of dye-house effluents, transforming two of the regulated contaminants into harmless salts which can safely be released.
Production of food and beverages
In the process of making beverages and food Ion exchange resins play an essential function. Breweries and soft drinks plants rely on them for the removal of Ca²⁺ as well as Mg²⁺, which can cause unintentional flavor changes. They also remove organic acids, thereby improving the stability of final products.
Conclusion
Water softeners that use ion exchange and Ion Exchange technology help solve important water quality problems. From softening the water in your home to making ultra-pure water for electronics their reversibility, selectivity and efficacy are the foundation for modern-day water treatment. If you’re dealing with limescale at home, or in industry-wide water purification knowing Ion Exchange can help you pick the most appropriate solution to clean high-performance, high-quality water.
For industrial-scale hard water problems Molewater’s Industrial Water Softener stands out–it makes use of advanced ion exchange techniques to effectively remove Ca²⁺ as well as Mg²⁺ to ensure the stability of equipment operation while meeting various industrial water requirements.