Water is indispensable in pharmaceuticals, used from drug R&D labs to commercial production. Unlike daily tap water, it requires pharmaceutical-grade water—prepared via specific processes to meet strict purity standards—with Water for Injection (WFI) as a key type. As a critical “raw material” for pharmaceutical safety and efficacy, WFI production meets the industry’s high water demands. Molewater, specializing in pharmaceutical-grade water, offers compliant solutions via advanced engineered processes. This article starts with WFI’s definition, analyzing its uses, differences from purified water, and production to highlight its pharmaceutical value.
What is Water for Injection (WFI)?
Water for Injection is widely used as a solvent in the pharmaceutical industry. It is used primarily to dilute injectable drugs and formulate injectable solutions. This directly affects drug safety and application. To meet the strict requirements of global pharmaceutical pharmacopoeias, such as USP, Ph. Eur.) WFI is required to maintain chemical impurities at very low levels and eliminate biological contaminants, including microbes that can cause infection. WFI’s production and storage systems use continuous water circulation and high temperature treatment to achieve this high purity standard. High temperatures destroy microbial structures while the continuous circulation prevents bacteria growth from static water.cause contamination of drugs with microorganisms or heavy metals which can cause safety hazards.
What is Water For Injection Used For?
Water for Injection is a source of high-purity pharmaceutical water. Its extremely low impurities, microorganisms and contaminants make it a key ingredient in drug production, medical devices manufacturing and medical operations. The uses of this water can be divided into two categories: core applications and extended applications.
Fortifying Drug and Device Safety
1. Production and Dilution of Injectable Drugs
WFI is the main raw material used to make injectable drugs. It is used to make transdermal, intravenous, intramuscular, spinal, and cardiac injectables. It also acts as a sterile diluting agent for adjusting drug concentration. WFI is a sterile diluent that meets the global pharmacopoeia standard, which prevents impurities from causing adverse reactions.
2. Production of Implantable Medical Devices
WFI ensures sterility by cleaning and rinsing key links in the production of implantables such as artificial joints and cardiac Stents. WFI’s high-purity devices ensure safety because implants stay in the body for a long time. Residual microbes and impurities can cause tissue rejection or infection.
3. Hemofiltration Medical Operations
In hemofiltration therapy, WFI has two key roles: it helps filter blood metabolic wastes and prepares/injects sterile replacement fluid to maintain patients’ fluid balance. With strict water quality demands, WFI stops contaminants from entering the bloodstream and avoids therapy-related complications.
4. Cleaning of Laboratory and Production Equipment
WFI cleans injectable container (vials and ampoules) as well as process equipment (filling machine, liquid tanks). It removes all residual drugs and detergents, keeps the equipment sterile, prevents cross contamination, and ensures that subsequent drugs are of high quality.
Covering Multi-Scenario High-Purity Needs
Beyond core uses, WFI fits other scenarios:
- it cleans drug-contacting caps, stoppers, and storage equipment as a cleaner;
- aids in making eye care products (e.g., artificial tears) and inhaled drugs to avoid sensitive part irritation;
- provides sterile water for cell culture media in biopharmaceuticals;
- and rinses biotherapy bioreactors or prepares liquids after solute supplementation, meeting diverse production needs.
How is WFI Produced?
The production of Water for Injection (WFI) requires a precise and complex process. Three commonly used methods are membrane separation systems, multi-effect distillation, and vapor compression distillation.
1. Membrane Separation Systems
Membrane systems for WFI manufacturing include a variety of approaches, each with varying ownership costs. One method is to combine reverse osmosis with ultrafiltration. These systems are less expensive and take up less space. However, they also require more monitoring. They also carry a greater risk of contamination.
The purification process is a multi-step procedure. Water softeners eliminate water hardness while activated charcoal filters remove chlorine. Reverse osmosis is used to remove most of the dissolved particles, organics, and ions. The remaining dissolved ions are then removed by electrodeionization. Ultrafiltration removes any remaining endotoxins or biological contaminants.
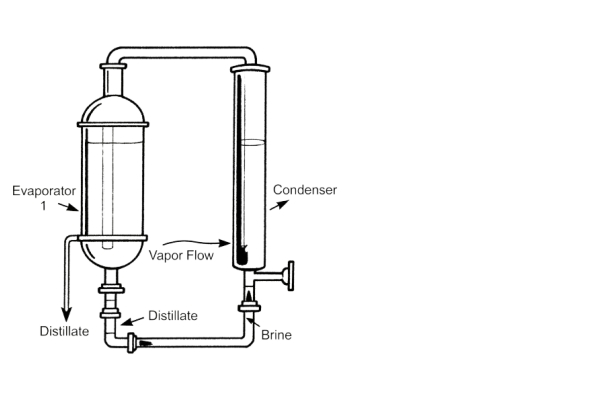
2. Multi-Effect Distillation
Multi-effect distillers produce WFI using multiple “effects.” Pharmaceutical multi-effect distillers feature a modular design and can optionally produce pure steam either separately or simultaneously. Standard features include:
- Dual condensers
- PLC-based control
- Hot standby option
- Vertical natural circulation evaporators
- Straight annealed tubes in evaporators
- PID loop control for first-effect pressure and feed water level
The unit comes fully assembled, wired, and piped at the factory.
3. Vapor Compression Distillation
In most cases, vapor compression distillation is used to produce pharmaceuticals. The feedstock for this process is usually water that has been dechlorinated and softened. This water boils in tube bundles. The steam is then passed through an oil mist separation device to remove any impurities. Pure steam is pumped into a compressor with a controlled saturation temperature and pressure. It’s then compressed to a higher saturation pressure.
Steam at high pressure and high temperature is discharged from the evaporator. It condenses along the outer walls of the tube bundles, and transfers the latent heat of vaporization into the boiling water within the tubes. The process is repeated, with new steam being generated. A distillate pump collects the condensed steam that has escaped from the tubes and sends it to a heat-exchanger. The heat exchanger is also used to discharge excess feed water, or blowdown. The heat exchangers cool the blowdown and distillate in their respective heat-exchangers, while also pre-heating the feed water. This helps reduce the energy consumption of the system.
Water for Injection vs. Purified Water
In pharmaceuticals, WFI and Purified Water are both pharmaceutical-grade but differ greatly in purity, applications, and production.
1. Purity Standards
Purified water meets the basic requirements of pharmacopoeia for microorganisms and chemical impurities. It allows trace amounts of dissolved organics/ions without endotoxin tests. WFI is more stringent: It requires complete removal of endotoxins (0.25 EU/mL), no microorganisms, almost all dissolved particles and stricter limits on conductivity (≤5.1 µS/cm at 25°C), TOC and conductivity.
2. Application Scenarios
Purified Water is used for oral/external preparations (e.g., oral liquids, ointments) or non-sterile equipment cleaning. WFI is only for scenarios involving direct human contact, such as injectable/ophthalmic preparation/dilution, implantable device cleaning, and hemofiltration, to avoid infections.
3. Production Processes
Purified Water uses processes like reverse osmosis or electrodeionization, no deep endotoxin removal. WFI requires extra steps (e.g., multi-effect distillation, ultrafiltration) on top of Purified Water production to remove endotoxins/microorganisms, meeting injection-grade standards.
Partnering with Molewater
Water for Injection is an important component in pharmaceutical production. There are multiple production methods available based on various factors such as capacity, infrastructure and risk, reliability of the facility, energy efficiency requirements, and water recovery needs. Molewater will help you determine the best WFI solution for your facility. Each system has pros and cons.

Molewater is a leader in the design, manufacture, and service of engineered systems and products for WFI, Purified Water and Ultrapure Water generation. We are a leader in the design and manufacture of water purification systems. Our advanced technology and experience allow us to deliver sustainable, valuable solutions. We welcome you to contact our team at Molewater for more information.